Synthesis, Spectral Characterization and Biological Evaluation of Chromium(III) Complexes of Schiff Base ()

1. Introduction
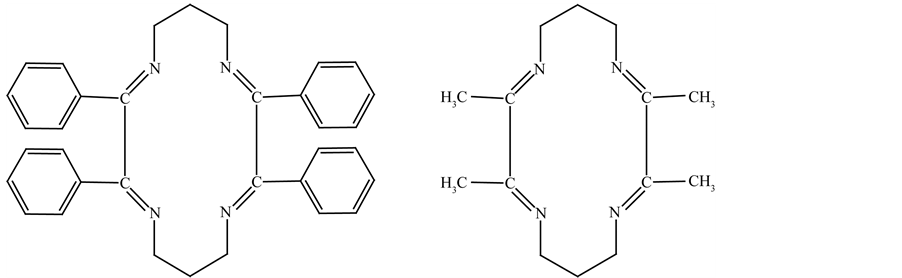
Azo Schiff base complexes contain both azo and azomethine groups. The azo group possess excellent donor properties and is important in coordination chemistry [1] -[3] and some azo compounds have shown to possess good antibacterial activity [4] [5] . Schiff bases are well known to have antifungal, antitumor and herbicidal activities [6] -[14] . Many metal complexes of naturally occurring porphyrins, corrins and phthalocyanines have been investigated because of their potential as dyestuffs or pigments. A Schiff base complex is of great importance [15] -[18] in enehancing various industrial applications and in a number of biological processes such as photosynthesis and dioxygen transport [19] . A number of reviews are available on the physiology and biochemistry of chromium in mammals [20] -[24] . In view of above applications it is highly desirable to synthesis and characterize the Cr(III) complexes with Schiff base. In view of the above in the present paper we report the synthesis and characterization of Schiff base complexes of chromium (III) with 2,3,9,10 tetraphenyl-1,4,8,11-tetraazacyclotetradeca-1,3,8,10 tetraene. (BPD) 2,4,10,12-tetramethyl-1,5,9,13-tetraazacyclohexadeca-1,4,9,12 tetraene. (ADP) 2,3,9,10 tetramethyl 1,4,8,11 tatraazatetredeea-1,3,8,10 tetraene (DDP) (Figures 1(a)-(c)).
2. Chemistry
All the chemicals used in the present work of high purity, Anala R grade and purchased from Sigma-Aldrich. Metal salts were purchased from E. Merck and used as received. The solvent used were either spectroscopic pure from SRL/BDH or purified by the recommended methods.
2.1. Preparation of Ligands
2.1.1. Preparation of 2,3,9,10 Tetraphenyl-1,4,8,11-Tetraazacdyclo Tetradeca-1,3,8,10 Tetraene. (BPD)
The ligand 2,3,9,10 tetraphenyl-1,4,8,11-tetraazacdyclo tetradeca-1,3,8,10 tetraene. (BPD) was synthesized by refluxing an ethanolic solution of 1,3 diaminopropane (0.5 mole) with an ethanolic solution of benzyl, (0.5 mole) in presence of ~3 mL of conc. HCl for 3 - 4 hours. The resulting mixture was kept overnight, when an off-white coloured crystalline compound separated. This was then filtered, washed with ethanol, and dried over P4O10. The ligand is soluble in water and melted at 218˚C.
 (a)
(a) (b)
(b)
Figure 1. Structure of ligands. (a) BDP; (b) ADP; (c) DDP.
2.1.2. Preparation of 2,4,10,12-Tetramethyl-1,5,9,13-Tetraazacyclohexadeca-1,4,9,12 Tetraene. (ADP)
2,4,10,12-tetramethyl-1,5,9,13-tetraazacyclohexadeca-1,4,9,12 tetraene. (ADP) was prepared by adding an ethanolic solution of acetylacctone (0.5 mole) to an ethanolic solution of 1,3 diaminopropane (0.5 mole) in presence of ~3 mL conc. HCl and the resulting solution was refluxed for 4 hours and then kept overnight. A white crystalline compound separated on filtration which was washed with ethanol and then dried over P4O10. The compound was soluble in most organic solvents, and it’s melting point was recorded as 226˚C.
2.1.3. Preparation of 2,3,9,10 Tetramethyl 1,4,8,11 Tatraazatetredeea-1,3,8,10 Tetraene (DDP)
2,3,9,10 tetramethyl 1,4,8,11 tatraazatetredeea-1,3,8,10 tetraene (DDP) ligand was synthesized by diactyl (0.5 mole) to an ethanolic solution of 1,3 diaminopropane (0.5 mole) in presence of ~3 mL conc. HCl and the resulting solution was refluxed for 4 hours and then kept overnight. Light-yellow crystals separated on filtration, which were washed with ethanol and then dried over P4O10. The ligand is water soluble and melts at 221˚C.
2.2. Preparation of Cr(III) Complexes with Ligands
The complexes with these ligands were synthesized by template method because the yield of the complexes was low when the ligands were treated with metal salt to form complexes.
A hot ethanolic solution of benzil/acetylacetone/diacetyl/ (0.01 mole) was added to an ethanolic solution of 1,3 diaminopropane (0.01 mole) and the resulting solution was refluxed for half an hour at ~40˚C. A solution of CrX3∙nH2O (0.005 mole) (X = Cl−, 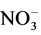 , NCS−) in ethanol and (X = 1/2
, NCS−) in ethanol and (X = 1/2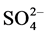 ) in water was then added to the above solution and refluxing was continued for a further four to six hours. On cooling the solution, a crystalline compound separated out. It was filtered, washed with ethanol and dried under vacuum over P4O10.
) in water was then added to the above solution and refluxing was continued for a further four to six hours. On cooling the solution, a crystalline compound separated out. It was filtered, washed with ethanol and dried under vacuum over P4O10.
2.3. Physical Measurement
The C, H and N were analysed in Carlo-Erba 1106 elemental analyzer. Molar conductance was measured on a ELICO (CM82T) conductivity bridge. Magnetic susceptibility was measured at room temperature, on CAHN- 2000 magnetic susceptibility balance using CuSO4∙5H2O as a calibrant. Infrared spectra of ligands and complexes were recorded as KBr pellets on a Perkin-Elmer 1310 spectrophotometer. The electronic spectra of complexes were recorded in DMSO, on a Shimadzu UV mini-1240 spectrophotometer. EPR spectra of complexes were recorded on JEOL, JES, FE3XG, EPR spectrometer. The spectra were recorded in solid as polycrystalline sample at room temperature on E4-EPR spectrometer using the DPPH as the g-marker.
3. Results and Discussion
On the basis of elemental analyses (Table 1) the complexes were found to have CrLX3 (X = Cl−, 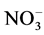 , NCS−) composition. Molar conductance, measured in DMF solution (Table 1) of these complexes indicates that these complexes are 1:1 electrolytes in nature, therefore, these complexes may be formulated as [CrLX2]X.
, NCS−) composition. Molar conductance, measured in DMF solution (Table 1) of these complexes indicates that these complexes are 1:1 electrolytes in nature, therefore, these complexes may be formulated as [CrLX2]X.
3.1. IR Spectra of Ligands
The IR spectra of the ligands show vibrations corresponding to azomethine groups. The bands at 1595, 1570, 1460 and 1405 cm−1 regions can be assigned to v(C=C) and v(C=N) skeletal frequencies while bands appearing at 960, 800, and 407 cm−1 may be assigned to ring breathing mode, C-11 deformation and C-C deformations respectively. The strong frequencies of ca. 1595 cm−1 are usually associated to v(C=N) coupled with phenyl ring vibrations. The band appearing at 1635 cm−1 may be assigned to symmetric and asymmetric v(C=N) vibrations respectively. The absence of bands in the range 3300 - 3200 cm−1 indicates that the amino group of 2,6 diaminopyridine and 1,3 diaminopropane have condensed with the diketone molecules. The presence of bands of ca. 1680 - 1670, ca. 1550, ca. 2920, ca. 1350, ca. 1265, ca. 1190 and ca. 680 cm−1 can be assigned to v(C=O), v(CO) + v(C=C), v(CH3) δsym (CH3), v(C-CH3), δ (CH) + c(C-CH3) and ring deformation modes respectively which indicates the presence of the diketone moiety in the ligands.
IR Spectra of the Complexes
Vibrations of free or coordinated C=O group or NH group at ca. 1680 - 1700 or ca. 1550 and ca. 670 cm−1 are

Table 1. Elemental analyses and molar conductance data of chromium(III) complexes.
not observed in any of the compounds The strong bands appearing as doublets around 1590 - 1620 cm−1 may be assigned to v(C=N) vibrations indicating the presence of coordinated azomethine groups. In these case ligands, phenyl ring absorptions appear in the 1600 - 1400 cm−1 region. Absorption bands in the region 900 - 700 cm−1 can be exclusively assigned to the imine and −CH2 absorptions of the macrocyles. IR spectral band at ~450 cm−1 is characteristic of metal ligand vibrations. The lowering of the v(C=N) band (1620 cm−1) indicates coordination through the nitrogen of v (C=N) group. The bands at 1620 cm−1 and ca. 840 cm−1 may be assigned to NH deformation coupled with NH out of plane bending.
The appearance of new bands at 1438 - 1430 cm−1 (v1), 1370 - 1375 cm−1 (V3), 1210 - 1215 cm−1 (v5), 1010 - 1015 cm−1 (v2) and 850 - 840 cm−1 (v6) show consistency with the monodentate nature of the nitrate group. The broad absorption band at 1405 cm−1 can be assigned to v3 of the uncoordinated nitrate group in the complex of ligand with Cr(NO3)3.
In thiocyanato complexes, strong bands are observed at ca. 2087 (v1) v(C=N), ca. 480(v2) NCS banding and ca. 778 cm−1 (v3), v(C=S) of the NCS group, respectively which are consistent with a monodentate N-bonded thioyanato group.
3.2. Electronic Spectra of the Complexes
The electronic spectra of all the above complexes show bands in the region 18,000, 22,000, 25,000 and 28,000 cm−1 which are consistent with the octahedral geometry Thus, an octaheadral may be assign to these complexes. Electronic spectra of the complexes were recorded in DMF. They display four bands at 17,465 - 18,800 (v1), 22,100 - 22,900 (v2). 24,200 - 25,680 (v3) and 27,700 - 29,400 cm−1 (v4). (Table 2). Six coordinate complexes with Oh symmetry show three spin-allowed bands [25] . Which the highest energy band assignable to the 4A1g ® 4A2g transition, occurs above 30,000 cm−1. The spectra of the complaxes under study show four bands below 30000 cm−1 which cannot be interpreted in terms of idealized Oh symmetry. The spectra can however be explained by assuming the presence of lower symmetry elements in the complexes, Such six-coordinated chromium can have either effective c4v or D4h symmetry. In the present complexes the four transitions observed can be assigned 4B1g ® 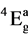 (v1), 4B1g ® 4B2g (v2), 4B1g ®
(v1), 4B1g ® 4B2g (v2), 4B1g ® 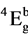 (v3) and 4B1g ®
(v3) and 4B1g ® 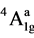 (v4) transitions arising from the lifting of the degeneracy of the orbital triplet (in octahedral symmetry) in the order of increasing energy and assuming the effective symmetry around the metal ion of D4h. In Oh symmetry (v1) and (v2) are derived from the 4T2g level, whilst v3 and v4 from 4T1g (F), The C4v symmetry has been ruled out because of the greater splitting of the first band.
(v4) transitions arising from the lifting of the degeneracy of the orbital triplet (in octahedral symmetry) in the order of increasing energy and assuming the effective symmetry around the metal ion of D4h. In Oh symmetry (v1) and (v2) are derived from the 4T2g level, whilst v3 and v4 from 4T1g (F), The C4v symmetry has been ruled out because of the greater splitting of the first band.
3.3. Magnetic Moment of the Complexes
The magnetic moment of these complexes, at room temperature (296 k), lie in the range of 3.71 - 3.84 B.M. as shown in (Table 2), which are close to spin only value of 3.86 B.M thereby, suggesting an octahedral geometry around the chromium ion.
3.4. EPR Spectra of the Complexes
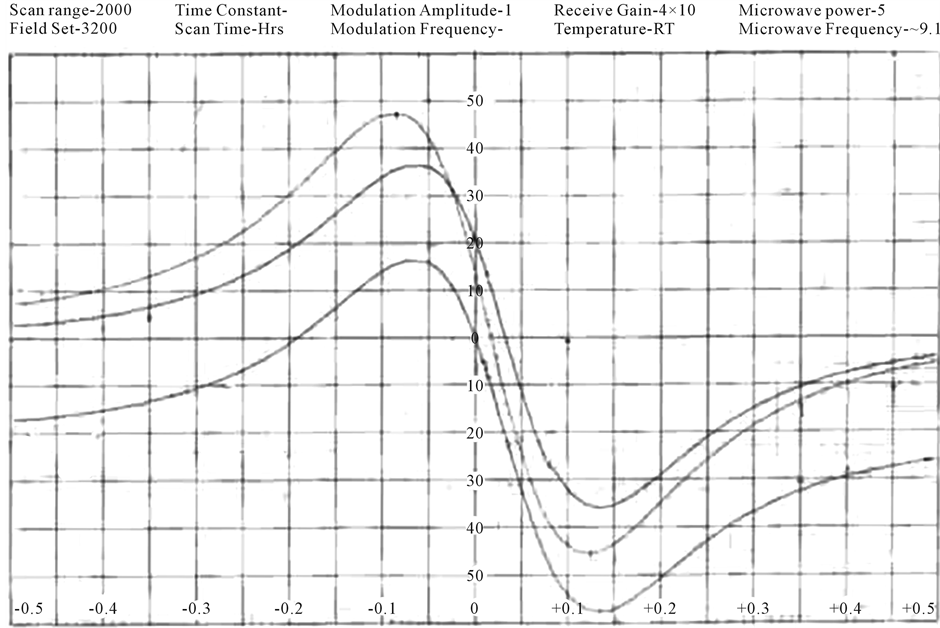
The EPR spectra of the polycrystalline samples have been recorded (Figures 2(a)-(c)) at room temperature. The “g” values lie in the range 1.98 - 2.01 (Table 3). The “g” values are calculated using the expression.
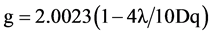
where λ is the spin-orbit coupling constant for the metal ion in the complex. Owen [26] noted that the reduction of spin orbit coupling from the free-ion value of 90 cm−1 for chromium (III) can be employed as a measure of metal-ligand covalency. It is possible to define a covalency parameter analogous to the nephclauxetic parameter which is the ratio of the spin-orbit coupling constant for the complex and the free Cr3+ ions.
Energy of the first spin allowed transition 4A2g ® 4T2g directly gives the value of 10Dq.
Spin Hamiltonian for Cr(III) complexes (S =3/2) may be written as

Table 2. Magnetic and electronic spectral bands of chromium(III) complexes.

Table 3. Ligand field parameters and ESR spectral data of chromium(III) complexes.
 (a)
(a)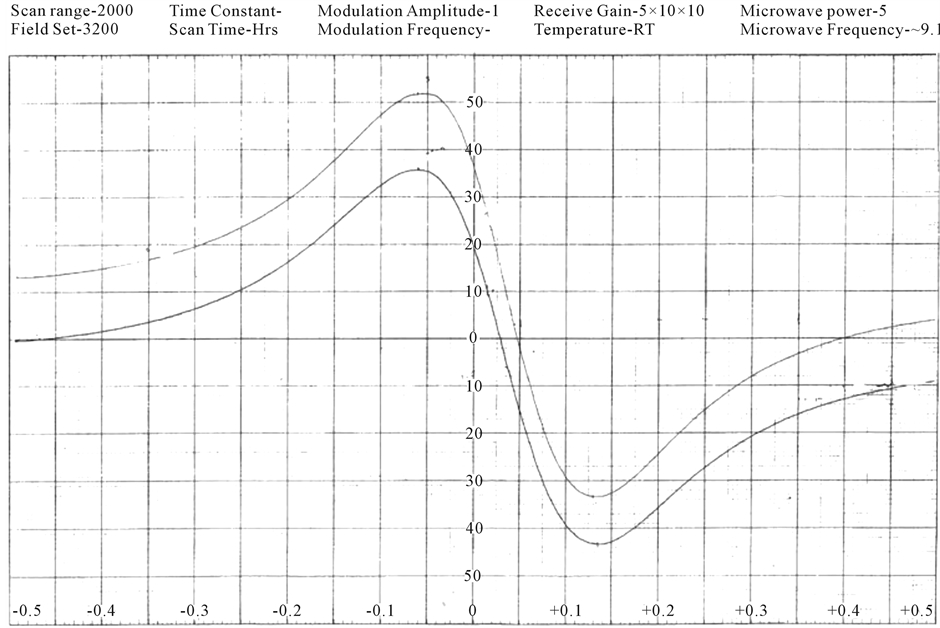 (b)
(b)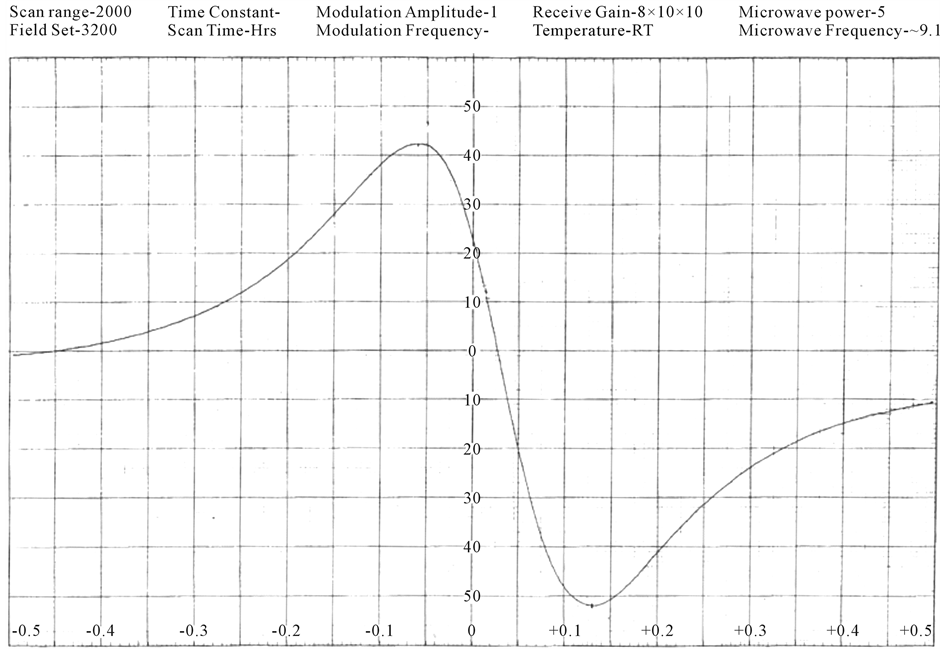 (c)
(c)
Figure 2. (a) ESR spectrum of [Cr(ADP)(NO3)2](NO3) complex; (b) ESR spectrum of [Cr(DDP)Cl2]Cl complex; (c) ESR spectrum of [Cr(DDP)(SCN)2](SCN) complex.
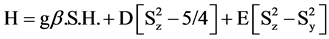
The 4F state of d3 ion in octahedral symmetry has the orbital singlet state lowest in energy with all excited states at much higher energies. Thus, the d3 ion has relatively long spin relaxation effects and gives narrow ESR absorption line, even at room temperature. In octahedral symmetry, ground state belongs to A2g irreducible representation and is connected through the spin-orbit coupling to the excited T2g state only, and so, the g and A terms are very nearly isotropic even in highly distorted crystal fields. In d3 ions the symmetry of the nearly field is primarily exhibited through spin-spin terms D and E.
Thus, on the basis of elemental analysis, molar conductance measurements, magnetic susceptibility measurements, ir, electronic. and esr spectral studies, the following structures may be proposed for the complexes. (Structure of complexes) (Figures 3(a)-(c)).
Ligand Field Parameters
Various ligand field parameters have been evaluated and are listed in Table 3 and Table 4. The energy of the first spin-allowed transition 4B1g ® 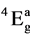 directly gives the values of 10Dq. B has been evaluated from the relation
directly gives the values of 10Dq. B has been evaluated from the relation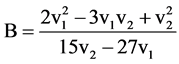
where v1 and v2 are the energies of the transitions 4B1g ® 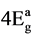 and, 4B1g ® 4B2g, respectively, the nephelauxetic paramter, β is readily obtained using the relation β = B (complex)/B (free ion), where B (free ion) = 918 cm−1. The results are presented in Table 3. The value of β lies in the range of 0.31 - 0.52 These values indicate that the complexes have appreciable covalent character.
and, 4B1g ® 4B2g, respectively, the nephelauxetic paramter, β is readily obtained using the relation β = B (complex)/B (free ion), where B (free ion) = 918 cm−1. The results are presented in Table 3. The value of β lies in the range of 0.31 - 0.52 These values indicate that the complexes have appreciable covalent character.

Table 4. NSH hamiltonian parameters of the chromium(III) complexes.
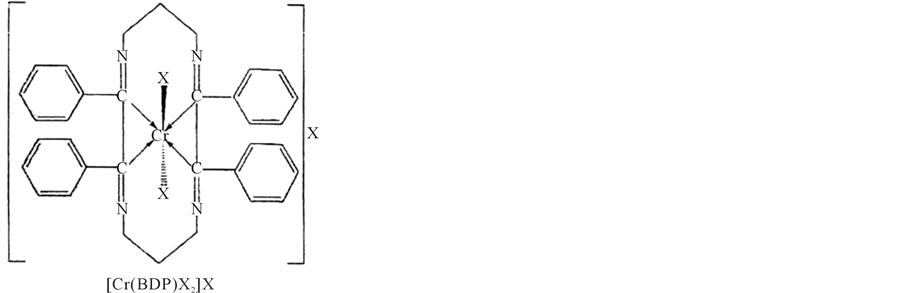 (a)
(a) (b)
(b)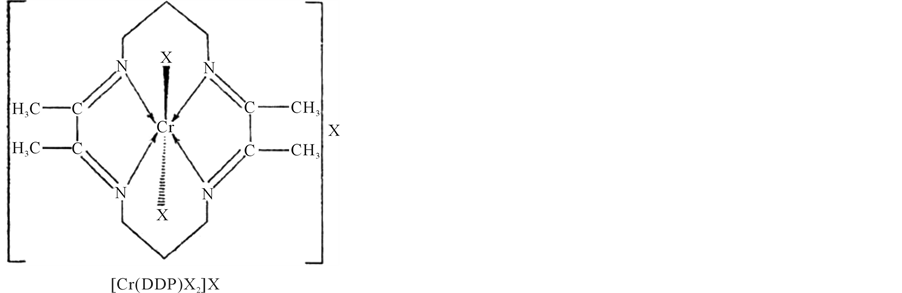 (c)
(c)
Figure 3. Structure of complexes.
According to jorgensen [27] for the 3d transition. B is well expressed by the relation B(cm−1) = 384 + 58q + 124 (Z + 1) − 540/(Z + 1)
From this relation the values of Z for the present complexes lie in the range 0.02 - 0.48 (Table 3) which is considerably below the format +3 oxidation state of chromium. Some other ligand field parameters have also been calculated (Table 3).
The transition v2 is equal to 10Dqxy and the saparation between v1 and v2 is of first order (35/4) Dt and Dt is related to the in-plane and out-of-plane field strengths as Dt = (4/7) [Dqxy and Dqz are in-plane (xy) and out-ofplane (z) Field strength, respectively. The radial parameter Ds has been ealculated from the splitting of
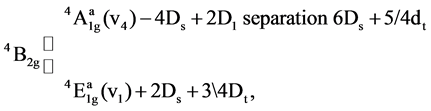
The values of these parameters (Table 4) are comparable to those observed for chromium complexes involving similar set of chromophore [28] . However, it may be pointed out that these parameters are not standardised and thus require modifications. To overcome the shortcoming of various parameters of the classical Hamiltonian for tetragonal complexes, Lever et al. [29] [30] had advanced the theory of a Normalised Spherical Harmonic (NSH) Hamiltonian. The NSH parameters DQ, DS, DT, DQxy, and DQz are fully capitalized to relate them to the corresponding crystal field parameters. Yet emphasize their distinction. The NSH classical parameters are related by,

There are several advantages of NSH Hamiltonian theory: 1) the theory takes into account an off-diagonal contribution to D1 2) DQ is a measure of the average ligand field experienced by the metal ion, unlike the classical Dq which is the measure of the in-plane field only and 3) the parameters of NSH theory are independent of the coordinate system used for the calculated and may be compared with the crystal field or angular overlap parameters DQxy (in plane field strength) and DQz (out-of-plane field strength) which are determined by the equations.
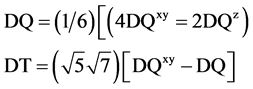
However it may be pointed out that these parameters have artificial significance. For DQ itself is a measure of average ligand field strength. Further, the ratio (DT/DQ) has been shown to be a good measure of the degree of tetragonal distortion. The values of (DT/DQ) lie in the range 0.033 - 0.034. These values are much lower then the limiting value (0.4226) for a square planar complex and suggest a small distortion from idealized cubic symmetry in these complexes.
4. Biological Study
The ligand (L) 2,3,9,10 tetraphenyl-1,4,8,11-tetraazacyclo tetradeca-1,3,8,10 tetraene. (BPD), ligand free metal ions and its complexes were evaluated against different species of bacteria and fungi as per the procedure reported earlier [31] -[34] . In both, antibacterial and antifungal studies ligand free metal ions in solution show inhibition capacity slighty more than the ligand but much less than complexes against all the species under study.
4.1. Antibacterial Screening
The compounds were screened against Sarcinalutea (gram-positive) and Escherchiacoli (gram-negative) bacteria, as growth inhibitor by disc diffusion technique [31] [32] . The results of the antibacterial screening show the maximum inhibition by the (CrL(NO3)]NO3 complexes.
4.2. Antifungal Screening
Aspergillus-niger and Aspergillus-glaucus fungi were used as the test organism for all the newly synthesized compounds for the purpose of antifungal screening by agar plate technique [33] [34] . All the complexes show nearly the same inhibition.
5. Conclusion
The present study revealed six coordinated octahedral geometry for the Cr(III) complexes. All the ligands act as a tetradentate manner coordinating through four nitrogons of the azonethine groups in an N, N, N, N fashion moreover, the fungicidal data reveal that the complexes were superior to the free ligand in the inhibition of the tested fungi it is proposed that concentration plays a vital role in increasing the degree of inhibition, the activity increased with increasing concentration of the complexes.
Acknowledgements
We are thankful to DRDO New Delhi for financial and I.I.T. Bombay for Recording EPR spectra.
NOTES
*Corresponding author.