
1. Introduction
Sparassis crispa is an edible mushroom with a number of reported medicinal benefits. This fungus is commonly known as cauliflower mushroom because the shape of its fruiting body closely resembles with cauliflower. It is also called brown rot fungi because it causes brown rot and butt rot in living conifers [1] . It is called by different names in different countries. For instance, in France it is called by the names: Clavaire crepue, Morille des pins, Sparassis crepue and Crete de coq; in Germany they call it Feisterling digal; Sheikaosin in China and in Japan “Hanabiratake” and “Hanabiramaitake” because it grows on larch trees [2] . As reported by Kimura [3] , the fruit body of S. crispa has about 90% water and contains 13.4 g protein, 2.0 g fat, 21.5 g carbohydrate, 1.8 g ash and 61.2 g dietary fibre per 100 g of dry weight. This fungus belongs to the phylum; Basidiomycota, class; Agaricomycetes, order; Polyporales, family; Sparassidaceae, genus; Sparassis and species; crispa [3] . The content of D2 Vitamin, that is helpful in intestinal calcium absorption, is about 0.17 mg per 100 g of dry weight [2] . The results obtained from headspace analysis indicate that 3-octanone, DL-3-octanol, and 1-octen-3-ol contribute to the particular aroma of this mushroom [4] . Medicinal properties in S. crispa include anticancer activities‚ antiangiogenic‚ anti-metastatic, wound healing and immune stimulation effects [5] -[7] . It has been recorded that β-glucan content of S. crispa is about 43.6% as measured by enzyme method by Japan Food research laboratories. Thus, S. crispa can be utilized for mass production of β-glucan [8] . Kimura [3] reported that the medicinal value of this mushroom is mainly due to 6-branched 1‚3-beta-glucan (SCG).
High fruit body yield is one of the major desirable outputs of mushroom cultivation. Among other methods, some researchers have tried to improve the fruit body development and yield of edible mushrooms by application of electric pulse stimulation. For example, Tsukamoto et al. [9] found that the yield of Shiitake from logs to which the pulsed high voltage was applied was twice as much as that from logs in which the pulsed voltage was not applied. Application of high voltages of up to 120 kV in bottle cultivation of Hypsizygus marmoreus and Pleurotus eryngii increased the fruit body yield of these mushrooms by 13 - 15 percent [10] . Islam and Ohga [11] reported a significant increase in fruit body yield of Tricholoma matsutake as a result of applying electric pulse. Having seen that this technology has worked with success in a number of mushrooms, a study was conducted to investigate the effect of electric pulse stimulation on fruit body development and yield of SC-1 strain of S. crispa. Prior to this, we also carried out experiments to determine the effect of environmental and nutritional factors on mycelial growth of SC-1 strain of S. crispa.
2. Materials and Methods
2.1. Strain Used
The strain SC-1 of S. crispa, accession number KUMB 1201 in the Mushroom Culture Bank at the Laboratory of Forest Resource Management, Kyushu University was utilized for this study. This strain was isolated from the fruit body of naturally growing S. crispa in Miyazaki Research Forest of Kyushu University (Figure 1).
2.2. Effect of Temperature
To investigate the favourable temperature for optimum mycelial growth of S. crispa (SC-1), five different temperatures were tested in PDA medium. The medium was autoclave sterilized for 15 minutes at 121˚C. Soon after sterilization the medium was poured into petri dishes. Each plate was filled with about 25 ml of PDA. Agar plugs (5 mm diameter) with actively growing mycelium were inoculated by placing on the centre of the medium surface in each petri dish. Four inoculated plates were incubated at each of the following temperatures: 15˚C, 20˚C, 25˚C, 30˚C and 35˚C. The measurements of radial mycelial growth were done using the method as described by Chioza and Ohga [12] . The diameter of each petri dish was determined by calculating the average of three diameter measurements.

Figure 1. Sparassis crispa growing naturally on a Pinus densiflora base in Miyazaki Research Forest of Kyushu University.
2.3. Effect of pH
The PDA medium was prepared in five flasks which were adjusted to pH 5, 6, 7, 8 and 9 before sterilization. The pH adjustments were made by using 1 N NaOH or HCl. After autoclaving for 15 minutes at 121˚C, media were poured into petri dishes with about 25 ml of PDA each. Agar plugs (5 mm diameter) with actively growing mycelium of S. crispa (SC-1) were inoculated on the plates as described earlier. Inoculated petri dishes were incubated for 28 days at 25˚C. The measurement of mycelial growth was made using the method explained above.
2.4. Screening of Carbon Source
To investigate the favorable carbon source(s) for mycelia growth of S. crispa (SC-1), experiment was conducted by using a basal medium along with 10 carbon sources namely fructose, glucose, lactose, galactose, maltose, mannose, sorbitol, sucrose, xylose and xylitol. The basal medium was composed of 0.05 g MgSO4, 0.46 g KH2PO4, 1.0 g K2HPO4, 120 µg thiamine-HCL, 20 g agar and 1000 ml distilled water. Each carbon source, together with 5 g of peptone, was added to the basal medium at the concentration of 0.1 M [13] . The media were sterilized for 15 minutes at 121˚C before inoculating each plate with agar plugs (5 mm diameter). The plates were then incubated at 25˚C for 28 days. On the 28th day after incubation colony diameters were measured. Diameter for each plate was recorded as an average of three diameter measurements [12] .
2.5. Screening of Nitrogen Sources
The basal medium used for screening nitrogen sources was the same used for screening the carbon sources. The following 10 nitrogen sources were tested: alanine, urea, glycine, calcium nitrate, potassium nitrate, methionine, histidine, arginine, ammonium phosphate and ammonium acetate. Each nitrogen source was added with 20 g of glucose to the basal medium at the concentration of 0.02 M as described by Shim et al. [14] . The basal medium was adjusted to pH 6 before autoclaving at 121˚C for 15 minutes. All petri dishes were incubated for 28 days at 25˚C. On the 28th day after incubation, colony diameters were measured as explained earlier.
2.6. Electric Pulse Stimulation
2.6.1. Culture Media and Growth Conditions
Bottle cultivation method was used in fruit body production of S. crispa. Each bottle contained 700 g of substrate (larch sawdust, wheat bran and corn powder in the ratio 5:1:1). The medium was sterilized by autoclaving at 120˚C for 30 minutes and then cooled down to room temperature. The sawdust spawn was inoculated by spreading evenly on the surface of the substrate in the bottle. The inoculated bottles were then incubated at 25˚C, in the dark, until full colonisation. In order to induce fruit body formation, kinkaki treatment (removal of spawn and the uppermost part of the medium) followed by chilling procedure were applied.
2.6.2. Electric Pulse Treatment
Electric pulse treatments were applied immediately after exposing the fully colonised bottles to chilling. Pulsed power voltages of 100, 120, 130 and 170 kilovolts were directly applied through the electrodes from the instrument to the fully colonised substrate in the bottles. The instrument (YH1G-200K-1KJC) used was manufactured by Yamabishi Electric Co. Ltd., Japan.
2.7. Data Analysis
Determination of statistical differences was done by Analysis of Variance (ANOVA) followed by Tukey’s test (p < 0.05). Minitab 16 statistical software (Minitab Inc.) was used to perform the analyses. Data in all tables is presented as mean plus or minus standard deviation.
3. Results and Discussion
3.1. Effect of Temperature
The most suitable temperature for mycelial growth of S. crispa was found to be 25˚C (Figure 2). This temperature is reported as the most favourable for the majority of mushrooms which include Coprinus comatus [15] , Paecilomyces hepiali [12] , Paecilomyces fumosoroseus [14] [16] , Ophiocordyceps heteropoda [17] and Cordyceps cardinalis [18] . The next favourable temperature was 20˚C. Cheong [19] reported 23˚C as the optimum temperature for mycelial growth of S crisp. This finding is within the favourable range (20˚C - 25˚C) obtained in this study. Shim et al. [1] reported that mycelial growth of S. crispa decreased with temperatures above 25˚C. That is similar to the findings of this study. At 30˚C growth was considerably slow (Figure 2) and there was no growth at 35˚C. Apart from few mushrooms such as Volvariella volvacea [20] and Macrolepiota procera [21] , the majority do not have mycelial growth at temperatures beyond 30˚C. Denaturation of important ezymes which catalyse fungal metabolic processes may be the main reason for not having any growth at 35˚C [22] .
3.2. Effect of pH
This study shows that slightly acidic to neutral initial agar media pH is most favourable for mycelial growth of S. crispa (Figure 3 and Figure 4). The PDA medium with initial pH of 6 resulted in highest mycelial growth among the pH levels tested (pH 5 - 9). However, mycelial growth measurements within the initial pH range 5 - 7 were not statistically different. The colony morphological characteristics among this pH range were also quite similar (Figure 4). This study confirms the finding by Cheong [19] in terms of the most favourable initial media
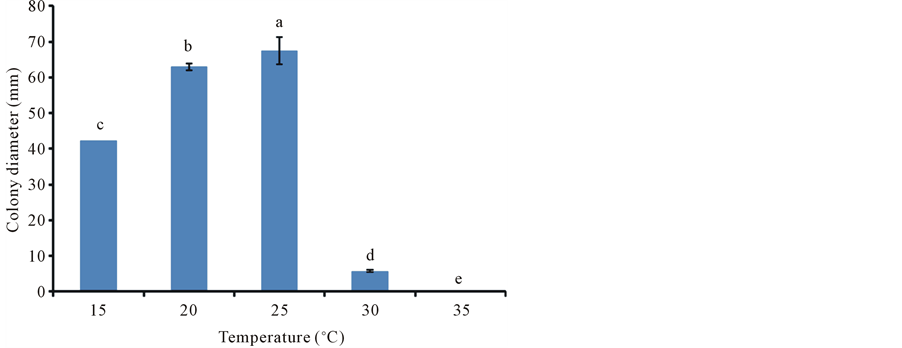
Figure 2. Mycelial growth of S. crispa at different temperatures in 28 days. Graph bars with different letters at the top represent values that are significantly different according to Tukey’s Test (p ˂ 0.05). The error bars represent standard error.

Figure 3. Mycelial growth of S. crispa at different pH levels on 28th day (Incubation temperature: 25˚C). Graph bars with different letters at the top represent values that are significantly different according to Tukey’s Test (p ˂ 0.05). The error bars represent standard error.

Figure 4. Colonies of S. crispa after 28 days of mycelial growth in media with different initial pH levels (Incubation temperature: 25˚C).
pH for mycelial growth of S. crispa. In media with initial pH levels beyond 7, there was a sharp decline in mycelial growth. This indicates that this fungus does not favour media with alkaline initial pH.
3.3. Effect of Carbon Source
In order to determine suitable carbon sources for mycelial growth of S. crispa, ten different carbon sources were tested. Among them, fructose followed by glucose supported the best mycelial growth (Table 1). The most unsuitable carbon source was galactose which supported no mycelial growth at all (Table 1). The colony diameter measurements from fructose and glucose, 36.9 ± 1.9 mm and 31.6 ± 6.8 mm respectively, were statistically the same and significantly much higher than the rest of the carbon sources which supported no more than 6.9 mm of

Table 1. Effect of carbon sources on mycelial growth of S. crispa in the basal medium.
Colony diameter values that do not share a letter are significantly different according to Tukey’s Test (p < 0.05). The basal medium was composed of 0.05 g MgSO4, 0.46 g KH2PO4, 1.0 g K2HPO4, 120 µg thiamine-HCl, 20 g agar and 1000 ml distilled water. Each carbon source was added to the basal medium at the concentration of 0.1 M. The colony diameter was measured after 28 days of mycelial growth.
colony diameter growth in 28 days. This study shows that this fungus has preference for simple monosaccharides. The reason could be that they can be easily absorbed and metabolized during cellular respiration. Our finding differs with that of Shim et al. [1] on galactose for the same mushroom. They reported growth of about 7.6 mm/15 days on galactose as carbon source while in this study no growth was observed on the same.
3.4. Effect of Nitrogen Source
Out of the ten nitrogen sources tested, the most favourable was found to be glycine followed by alanine (Table 2). Shim et al. [1] also found the highest mycelial growth of S. crispa when cultural media was supplemented with glycine. There was no growth in media with histidine, arginine, ammonium phosphate and ammonium acetate as nitrogen sources. This is in agreement with findings of Shim et al. [1] on ammonium acetate and histidine. However, on arginine and ammonium phosphate the results differ in that they found mycelial growth when these compounds were used as nitrogen sources. In our study urea supported some mycelial growth whereas Shim et al. [1] observed no growth with the same compound. This may be due to differences in the strains used.
3.5. Effect of Electric Pulse Stimulation
Results showed that an electric pulse has a significant effect on fruit body yield of S. crispa. As the voltage increased, the fruit body yield also increased (Figure 5). In the case of dry weight yield, it was observed that it increased more with the increase in voltage of the electric pulse between zero and 120 kV than between 120 kV and 170 kV. Fresh weight yield increased more with the increase in voltage between zero and 130 kV of electric pulse than between 130 kV and 170 kV. The highest yield was found at 170 kV of electric pulse. There was more primodia on substrate treated with electric pulse as compared to control. The percent increases of fresh weight yield from control on 100, 120, 130, and 170 kilovolts were 36%, 44%, 75% and 81% respectively. As regard to dry weight yield, the percent increases from control on 100, 120, 130, and 170 kilovolts were 27%, 54%, 63% and 67% respectively. Takaki et al. [23] reported 86% increase in the production of L. edodes by applying electric pulse. Two possible explanations have been reported for the increase in fruit body production of mushrooms as a result of application of electric pulse. The first one is that the high voltage causes physical damage to the hypha and that stimulates fruit body formation [24] . The other is that some enzymes which result into development of fruit bodies may be activated by the electric pulse stimulation [25] .
4. Conclusion
This study has established that temperature preference for mycelial growth of SC-1 S. crispa strain is the same

Table 2. Effect of nitrogen source on mycelial growth of S. crispa.
Colony diameter values that do not share a letter are significantly different according to Tukey’s test (p < 0.05). The basal medium was composed of 0.05 g MgSO4, 0.46 g KH2PO4, 1.0 g K2HPO4, 120 µg thiamine-HCl, 20 g agar and 1000 ml distilled water. Each nitrogen source was added to the basal medium at concentration of 0.02 M. The colony diameter was measured after 28 days of mycelial growth.
 (a) (b)
(a) (b)
Figure 5. Effect of electric pulse stimulation on (a) fresh and (b) dry fruit body yields of Sparasia crispa.
as the majority of mushrooms, which is around 25˚C. The best pH range for vegetative growth of this fungus is 5 - 7. It has been shown that alkaline condition of the medium prior to sterilization is not suitable for this fungus. In case of carbon sources, fructose and glucose showed excellent results. Among nitrogen sources studied, glycine and alanine were found to be most preferred. Results on effect of electric pulse stimulation on fruit body yield of S. crispa have confirmed that fruit body yield of some mushrooms could be increased with application of electric pulse.