Evaluation of larvicidal efficacy and phytochemical potential of some selected indigenous plant against Anopheles gambiense and Culex quinquefasciatus ()
1. INTRODUCTION
Mosquitoes transmit serious human diseases, causing millions of death every year. Such diseases include malaria, filariasis, Japanese encephalitis, dengue, hemorrhagic fever etc. Malaria as a parasitic infection causes enormous medical, economic, and emotional burden in the world. Malaria continues to be a major cause of morbidity and mortality in tropical countries. It has been estimated that more than 300 - 500 million people are affected by malaria throughout the world [1]. About 90% of all malaria death in the world today occurs in Africa and south of Sahara. An estimated one million people in Africa die of malaria each year and most of these are children under the age of 5 years [2,3]. Although insect born diseases currently represent a greater health problem in tropical and subtropical climates, no part of the world is immune to their risks. Despite extensive control efforts, the incidence of the disease is not decreasing especially in developing countries where malaria remains a parasitic disease that causes major public health problem [4]. One of the approaches for the control of mosquito borne diseases is the interruption of disease transmission by killing or prevention of mosquitoes from biting human beings.
Herbal products with proven potential as repellants can play an important role in the interruption of mosquito borne disease at both the individual and community level. However, the discovery, development and the use of synthetic chemicals with persistent residual action have not only overshadowed the use of herbal products against mosquito but has also become the major weapon for mosquito control. The repeated use of these synthetic insecticides produces widespread insecticide resistant mosquitoes, causes undesirable effect on non-target organism, pollutes the environment and poses health risk to man [5, 6]. This has necessitated the need for research and development of environmentally safe, biodegradable, low cost indigenous methods for vector control which can be used with minimum care by individuals and communities in specific situations [7]. The control of mosquito at the larval stage is necessary and efficient in integrated mosquito management [8].
The aim of this work was to evaluate the larvicidalefficacy and phytochemical potential of four selected indigenous plant species; Annona squamosa, Citrus sinensis, Cymbopogon citratus and Azadirachta indica against Anopheles gambiense and Culex quinquefasciatus.
2. MATERIALS AND METHODS
2.1. Sample Collection and Identification
The plants; namely the leaves of Azadirachta indica, the peels of Citrus sinensis were locally collected from different locations within the premises of Federal University of Technology Owerri while that of Cymbopogon citratus and the leaves of Annona squamosa were collected from Eziobodo village Owerri West Local Governemt Area of Imo state, Nigeria. The plants were identified and confirmed at the Herbarium of the Department of Botany, Lagos State University, Lagos, Nigeria.
2.2. Sample Preparation and Extraction
Fresh leaves of each sample was washed, air-dried at room temperature and then ground into powder form before maceration with 95% ethanol for three cycles; each cycle involves soaking for three days at room temperature. The extracts were filtered and concentrated using a rotary evaporator (buchi, Switzerland) under reduced pressure at 40˚C to yield a concentrated ethanol extract. The aqueous extract was prepared by further soaking of the residue from the previous filtration step in ultra-pure water for 24 h and filtered again. The plant extracts were freeze-dried (labconco, USA) and stored dry in a refrigerator at 4˚C until used for further experiments.
2.3. Rearing of the Mosquito Species
The eggs of species of Anopheles gambiae and Cullex quinquefasiciatus were maintained in the mosquitoerearing laboratory of National Arbovirus and Vector Research Centre Enugu (NAVRC) Enugu state, Nigeria and reared in white basins containing tap water and maintained between 27˚C and 29˚C. When the eggs hatched into first instar larve, they were fed with yeast powder and biscuit powder in the ratio of 1:3. The larvae were reared until the fourth instar larvae emerged on the sixth day.
2.4. Phytochemical Analysis/Screening of Plant Extracts
The active principles were tested according to the methods used by [9].
2.5. Flavonoids
Dilute ammonia (5 ml) was added to a portion of ethanol filtrate of the extract. 1 ml concentrated sulphuric acid was added and observed for yellow colouration that disappears on standing. The presence or absence of flavonoids was noted and recorded.
2.6. Tannins
About 0.5 g of the plant extract was boiled in 100 ml of water in a test tube and then filtered. 10 drops of 1% ferric chloride was added and observed for a brownish green or blue black colouration. The presence or absence of tannins was noted and recorderd.
2.7. Saponins
5 ml of distilled water was added to 0.5 g of the extract in a test tube. The solution was vigorously shaken and observed for a stable persistent froth. The presence or absence of saponins were observed and recorded.
2.8. Cardiac Glycosides
1ml of lead acetate was added to 2 ml of plant extract, shaken and filtered. The filtrate was extracted in an equal volume of chloroform. The chloroform layer was evaporated to dryness in a dish over water bath. The residue was dissolved in 3 ml of 3.5% ferric chloride in glacial acetic acid and left to stand for one minute.1ml of concentrated H2SO4 was ran down the sides of the test tubes and observed for a blue colouration at the interface which is a positive test for de-oxy sugars. The presence or absence of cardiac glycosides was noted and recorded.
2.9. Alkaloids
1 ml of plant extracts was shaken with 5 ml of 2% HCl and heated in a steam bath and filtered.1ml of the filtrate was heated with 0.5 ml of Wagner’s reagent and observed for reddish brown precipitate. The presence or absence of alkaloids was noted in the extract.
2.10. Larvicidal Bioassay
The bioassay was performed at a temperature of 27˚C, relative humidity of 70% - 80%, photoperiod of 12:12 (light:dark) and pH 7.0 of distilled water. The test concentrations used for larvicidal bioassay were 5 mg/ml, 10 mg/ml, 20 mg/ml, 30 mg/ml and 40 mg/ml of each extract. Each of the individual plant extracts were weighed according to required concentration and dissolved in 2 ml of ethanol. 95 ml of distilled water was measured and poured into each of the containers to be used. The test concentrations dissolved with ethanol were introduced into the containers containing 95 ml of distilled water. 10 of the 4th instar larvae of the mosquitoes were selected and counted using micropipette and put into small bottles and made up to 3 ml mark with distilled water and were introduced into the containers.
A control was also maintained by adding 2 ml of ethanol to 95 ml of distilled water and 10 of 4th instar larvae. 3 ml of distilled water was later introduced. The larvae were fed with yeast powder and biscuit powder at the ratio of 1:3 on daily basis (sprinkled on the surface of the water).The larval mortality were counted and recorded in percentages at 24, 48 and 72 hrs intervals. Dead larvae were removed to avoid decomposition.
3. RESULTS
Results showed that ethanol extracts of Citrussinensis and that of Cymbopogoncitratus at 20 mg/ml recorded the highest mortality at 48 and 72 hrs of exposure followed by Azadirachta indica against Anopheles gambia. The result also showed that at 40 mg/ml, all ethanol leaf extracts showed maximum mortality against Anopheles gambiae larvae after 72 h exposure period (Table 1). The dose dependent larvicidal effect of aqueous leaf extracts of Citrussinensis and that of Cymbopogon citratus recorded the highest percentage mortality at 72 hrs of exposure followed by the Azadirachta indica and Annona squamosa (Table 2).
From the results presented in Table 3, it can be observed thatethanol extract of Citrus sinensis at 40 mg/ mlexerted the highest larvicidal effect against Culex quinquefasciatus at various exposure time.
Results from Table 4 showed that at 40 mg/ml, there was maximum mortality of Culex quinquefasciatus by aqueous leaf extracts of Azadirachta indica, Cybopogon citrates, Citrus sinesis and Annona squamosa after 72 h.
The results presented in this work showed that ethanol extracts of all plant samples had greater larvicidal effect against Anopheles gambiense and Culex quinquifasciatus than aqueous extracts. The phtyochemical analysis showed that the four plants were positives for flavonoids, while Azadirachta indica and Annona squamosa where found positive for saponin. Citrussinensis, Cymbopogon citratus and Annona squamosa were positives for alkaloids while Citrus sinensis and Azadirachta indica were found positives for tannins (Table 5).
4. DISCUSSION
Mosquito borne disease is one of the world’s most threatening problems. Anopheles gambiense and Culex quinquefaciatus are very important disease vector transmitting the arbovirs causing malaria and filariasis


Table 1. Mean larval mortality (%) of ethanol extracts of Azadirachta indica, Cybopogon citrates, Citrus sinesis and Annona squamosa against Anopheles gambiae 4th instar larvae.
respectively. Several studies have been carried out to investigate means of eradication of mosquitoes, primarily at the larval stage which proves to be more efficient than controlling mosquitoes themselves. The use of herbs presents a better option in comparison to chemical pesticides for the control of mosquito larvae, as chemicals present environmental hazards [8,10,11]. Several workers have suggested various larvicidal plant species in

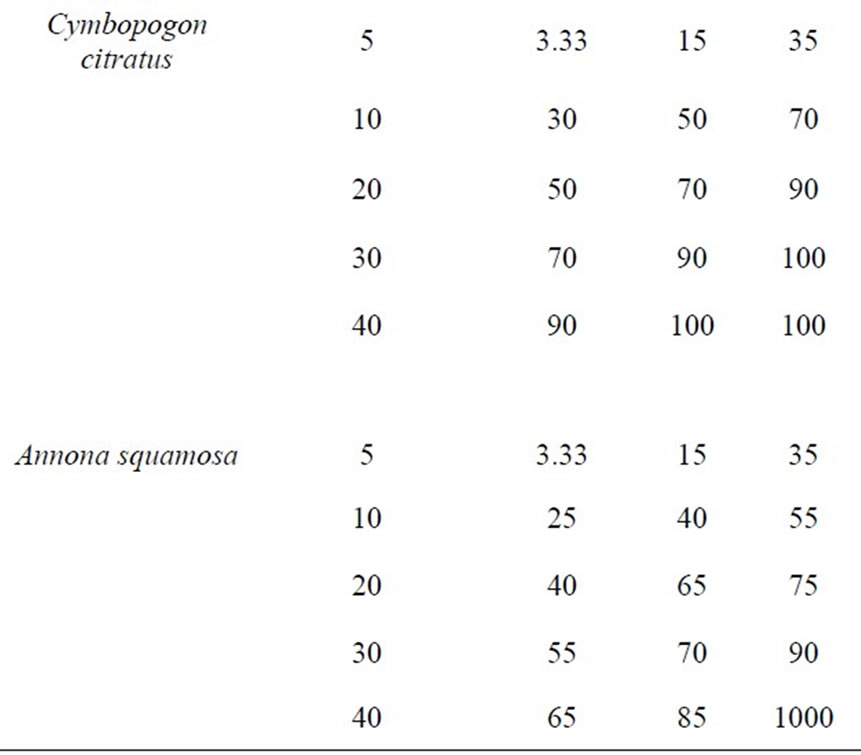
Table 2. Mean larval mortality (%) of aqueous extracts of Azadirachta indica, Cybopogon citrates, Citrus sinesis and Annona squamosa against Anopheles gambiae 4th instar larvae.
the control of mosquitoes. The larvicidal effect of Annona squamosa and Cymobogon citrates reported in this work agrees with the findings of other researchers. [12] reported the larvicidal potential of crude acetone extract of the seed of Annona squamosa on the larvae of Culex quinquefaciatus. [13] reported comparative efficiency of Annona squamosa Linn, Pongamia glabra vent. and Azadirachta indica against mosquito vectors. [14] reported variations in toxicological efficacy with three


Table 3. Mean larval mortality (%) of ethanol extracts of Azadirachta indica, Cybopogon citrates, Citrus sinesis and Annona squamosa against Culex quinquefasciatus 4th instar larvae.
mosquito species to crude aqueous extract of fruit pods of Swartzia madagascariensis. [15] also reported larvicidal properties of Cymbopogon citratus on Culex quinquefaciatus after 72 h exposure time. Results have shown that the aqueous extracts of the test plants had less larvicidal effect compared to the ethanol extracts. [16] worked on the phytochemical component of Nigerian medicinal plants and reported the presence of tannins, saponin, flavonoids and other active componenet on Citrus sinensis, Occiumgriatisimum, Cymbopogon citratus.


Table 4. Mean larval mortality (%) of aqueous extracts of Azadirachta indica, Cybopogon citratus, Citrus sinesis and Annona squamosa against Culex quinquefasciatus 4th instar larvae.
Result showed that all plant samples were positive for flavonoids while Azadirachta indica and Annonas quamosa were found positive for saponin.
The result of this study has shown that the leaf extracts of Azadirachta indica, Citrus sinensis and Annona squamosa had greater larvicidal effect on both Anopheles gambiae and Culex quinquefasciatus. In conclusion, this study recommends the use of leave extract of Azadirachta indica, Citrus sinensis, Cymbopogon citrates and Annona squamosa as an alternative to chemical in-

Table 5. Qualitative phytochemical analysis of leaf extracts of Azadirachta indica, Citrus sinensis, Cymbopogon citratus and Annona squamosa.
secticides in the control of mosquitoes especially the Anopheles gambiense and Culex quinquefasciatus. However, further and detailed analysis should be carried out to isolate the active compounds and optimum dosage responsible for larvicidal activity.
5. ACKNOWLEDGEMENTS
The authors would like to thank staffs of National Arbovirus and Vector Research Centre Enugu (NAVRC) Enugu state East Nigeria for providing their facilities. We are also thankful for Dr Oyebanji of the Lagos State University for helping in the identification and confirmation of the plants.
NOTES