1. Introduction
Soil salinization is a severe problem that limits plant productivity. According to [1], salinity affects about 20% of the irrigated soils in the world, and sodic soils alone represent 3.4% of all emerged lands.
The genus Prosopis includes many important arboreal and shrub-like species present in high-salinity areas of North and South America [2,3], having the unique ability to fix nitrogen and grow in such habitats [4]. The spiny shrub Prosopis strombulifera (Lam.) Benth. [2] ranges from the Arizona desert (USA) to Patagonia (Argentina), and is particularly abundant in high-salinity areas of central Argentina (Córdoba and southwestern San Luis province). In these high-salinity soils, proportions of NaCl and Na2SO4 salts are generally similar, although Na2SO4 was up to three times more abundant in certain samples [5]. Most studies concerning salt tolerance of plant species have been based on experiments in which NaCl is the predominant salt, and injury symptoms are often ascribed to the toxicity of Na+ and Cl− ions. Relatively few studies have focused on the effects of Na2SO4 on plant growth and physiology, even if Na2SO4 is present at higher concentrations than NaCl in the soils and groundwater in many areas of the world [6-9]. For that reason, it is important to compare the effects of these two salts on plant growth, in order to better understand the physiological responses of plants to soil salinity.
Our previous studies have shown that P. strombulifera has considerable variability in the salinity responses, depending on the type of salt(s) used, and osmotic potential (Ψo) in the culture medium. Stimulation of shoot growth at Ψo values up to −1.9 MPa (500 mM) NaCl is an interesting halophytic response. These plants grown in an increasing gradient of NaCl (250 - 700 mM·L−1) do not develop salt glands in the leaves. Some tissues display vacuolization, and the root system undergoes precocious lignification and/or suberisation of endodermal cells, with Casparian strips found much closer to the root tip than in glycophytes. These plants can therefore more efficiently filter soil solution to prevent passage of excess ions into the xylem [10]. In contrast, P. strombulifera is much less tolerant of Na2SO4. Plants grown in the presence of this salt showed immediate and sustained reduction of shoot height and leaf number per plant, accompanied by senescence symptoms such as chlorosis, necrosis, and leaf abscission. Total and individual leaf areas were strongly affected by Na2SO4 treatment, with a reduction that reached 60% and 30% respectively, at high salinity [11]. Na2SO4 treatment also induced structural alterations in cells and tissues, with consequent changes in growth patterns at various levels of organization, and anatomical and histological differences in roots, stems and leaflets, compared to control plants or plants grown in high NaCl [12]. These results demonstrate that plant growth responses may vary depending on the anion associated with sodium.
Plants adaptation to environmental constraints such as drought or salinity requires transitory and long-term reductions of transpirational water fluxes, a physiological response that is mediated by both constitutive and induced genetic determinants [13]. Stomata plays a fundamental role in the regulation of transpiration because, based on their density at the leaf surface, and their closing mechanism in response to environmental stimuli, they can exert a tight control on plant water loss [14]. However, the relationship between stomatal functionality and plant water status is very complex and several factors are involved [15]. Many studies have shown that water deficit leads to an increase in stomatal density [16-19] and a decrease in stomatal size [17,20,21], indicating this may enhance the adaptation of plant to drought [22]. Plant survival also depends on the compromise between photosynthesis and transpiration, and seedling stomatal abundance and distribution are probably under selective pressure in natural environments [23].
The traits currently used to score stomatal abundance are stomatal index (SI), which measures the proportion of epidermal cells that are stomata and stomatal density (SD) or number of stomata per area unit. SI and SD are the results of cell division patterns and of cell differentiation and expansion during organ growth [24,25]. SD depends on stomatal number and on the size and number of nonstomatal epidermal cells, while SI depends solely on celltype proportion, regardless of cell size, and therefore both traits provide complementary information on final stomatal abundance and pattern [23].
The aim of this work was to investigate stomatal abundance and distribution of P. strombulifera plants in response to different water status when the plants are subjected to different salt treatments (NaCl, Na2SO4 and their iso-osmotic mixture). Knowledge of these morphophysiological aspects will enable to understand the adaptive and survival strategies developed by this halophyte in relation to the environment in which it develops, providing important information for future work in biotechnological areas.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
Prosopis strombulifera seeds were collected from an area in southwestern San Luis province, Argentina, located at 33˚43'S, 66˚37'W, altitude 400 - 500 m, with a temperate climate (average annual temperature 15˚C - 20˚C) (Figure 1). This is predominantly a Prosopis alba forest located in a saline depression between annual 300 to 400 mm isohyets in the Monte phytogeographic region [26]. The soil was saline-sodic with abundant calcareous material and moderate salinity, has a sandy-loam texture and pH 7.5 [27]. Chemical composition was determined according to [28] Peña Zubiate et al. (1998). Profile from 0 to 35 cm depth is presented in Table 1.
Pods were collected randomly from 100 plants in the population, and peeled. Seeds were visually selected on the basis of uniform size and generally good health, scarified with 98% H2SO4 (sulfuric acid) for 10 min, washed overnight under running water, rinsed in distilled water, and germinated in Petri dishes with two layers of water-saturated filter paper at 37˚C for 24 h [10]. Germinated seedlings with roots ~20 mm long were grown in hydroponic conditions in black trays (28 × 22 × 10 cm; 200 seedlings per tray) with 10% full-strength Hoagland’s solution. Seedlings were self-supported in small holes on the tray cover until the end of the experiment. The trays were placed in a growth chamber (Conviron E15, Con-

Figure 1. (A) Sampling site (near El Bebedero stream); (B) General view of salty soils in the sampling site; (C) P. strombulifera native plant in their habitat.

Table 1. Chemical composition of soil profile in the sampling area.
trolled Environments Limited, Manitoba, Canada) with a cycle of 16 h light (200 µmol∙m−2∙s−1) (28˚C): 8 h dark (20˚C), relative humidity 70%. After one week, the nutrient solution was changed to 25% Hoagland’s solution (osmotic potential (Ψo) = −0.11 MPa).
After this, nutrient solution was changed weekly to maintain an adequate nutrient availability. Aeration was provided by an aquarium tubing system with peristaltic pump; pH of all medium was 6. The experiment was performed four times (2 trays per treatment each time).
2.2. Salt Treatments
A simple randomized design with four treatments was used (Control, NaCl, Na2SO4 and NaCl + Na2SO4). After 21 days growth in Hoagland’s solution, salt treatment was initiated by adding NaCl and/or Na2SO4 (50 mM·L−1 and 38 mM·L−1, respectively) every 48 h until reaching final Ψo values of −1, −1.9 and −2.6 MPa for monosaline and salt mixture treatments (measured with a vapor pressure osmometer, Model 5500; Wescor Inc., Logan, UT, USA). The salt mixture solution was made by mixing equal volumes of the monosaline solutions of the corresponding osmotic potentials (Table 2). At each interval, 20 control plants (no salt added, Ψo = −0.11 MPa) and 20 treated plants were collected at random from each tray for their anatomical study.
2.3. Leaf Stomatal Density and Guard Cell Size
For stomatal and epidermal cell counts, a clear nail varnish was applied on the abaxial and adaxial surfaces of leaves from each treatment (without detaching the leaf

Table 2. Increasing salt concentrations obtained by sequential addition of pulses every 48 h. i.e. 4 pulses means 4 × 37.9 ml aliquot of Na2SO4 L−1 Hoagland solution.
from the plant) to obtain an epidermal impression. The adhesive was allowed to dry for 2 - 3 min before being gently peeled off with forceps. On the other hand, epidermal tissue was stripped from the adaxial and abaxial surfaces of leaf lamina. For this, leaves were previously submerged in KOH 10% solution and heated for 24 h at 70˚C. The sections were stained with safranin and mounted on a microscope slide and covered with a cover slip. The leaf replicas were examined under the light microscope with clear camera (Zeiss Standard 16) analyzing 5 microscope fields (0.25 mm × 0.25 mm) in each one of them [29] and photomicrographs were taken with a Zeiss Axiophot microscope with an equipment of image capture and digitalization AxioVision 4.3, with camera AxioCam HRc. For stomatal measurement Image-Pro plus 2.0 Program was used. Length, width of guard cell and total area of individual stomata (guard cell complex including pore) in both leaf surfaces were measured. The number of stomata and the number of epidermal cells were counted from five fields of view randomly selected (area: 0.02979 mm2) at 400× magnification. The number of stomata per field was converted to number of stomata∙mm−2. The stomatal index (SI) was calculated as the number of stomata (S) per unit area divided by number of stomata and number of epidermal cells (E) following the Equation (1) [30]:
 . (1)
. (1)
2.4. Transpiration
Transpiration was determined indirectly by recording changes in volume of culture solutions at defined Ψo values [31]. Plants (three per treatment) were inserted in the perforated rubber stopper of a transparent graduated cylinder, containing a defined volume of solution, which was then sealed with silicone. Plants were maintained at the same conditions as mentioned for the hydroponic cultures for 4, 8, 12, 24, and 28 h, and the volume of the solution consumed was measured. To calculate the leaf area, leaves of seedlings were cut, digitally scanned (Hewlett Packard scanner PSC 1410), and their area was determined using Image-Pro Plus (2.0) program [32].
2.5. Statistical Analysis
Data were analyzed using InfoStat Program [33]. Twoway general linear model ANOVA was used to determine the effect of each treatment at each osmotic potential. Thus, the factors considered for two-way ANOVA were osmotic potential (Ψo) (−1.0, −1.9, or −2.6 MPa), and salt treatment (control, NaCl, Na2SO4, salt-mixture). Normality was verified with the Shapiro-Wilk test. Homogeneity of variance was verified with Levenne test. When necessary, data were transformed to meet the assumptions of ANOVA. For cases in which normality and homogeneity of variance were not verified, the non-parametric Kruskall-Wallis test was used. Bonferroni test was used as post-hoc analysis to determine differences between means. P values < 0.05 were considered statistically significant.
3. Results
3.1. Epidermal and Stomatal Characteristics of P. strombulifera Seedlings
Leaves of P. strombulifera showed an amphistomatic structure. The stomata were 90% paracytic and 10% anisocytic, showed a dispersal distribution, and were absent on the veins. Stomata were more abundant in adaxial epidermis (Figure 2). Simple unicellular trichomes were present on the apical region of leaf blade, and some smaller trichomes in the apical region on the abaxial leaf surface.
3.2. Effects of Salinity on Stomatal Density, Epidermal Cell Density and Stomatal Index
Different salt treatments on P. strombulifera plants differently influenced leaf micromorphological traits. Stomatal

Figure 2. Microphotograps of abaxial and adaxial epidermis of 48 days-old P. strombulifera plants. Control (a), NaCl (b), Na2SO4 (c), NaCl + Na2SO4 (d) (Ψo = −2.6 MPa). Scale bar 40 µm.
density (SD) was not affected by different salt treatments at low salinity (Ψo = −1 MPa). However, in Na2SO4 treated plants a significant increase in SD at moderate and high salinity (Ψo = −1.9 and −2.6 MPa) was observed in both abaxial and adaxial leaf surface. NaCl and NaCl + Na2SO4 treated plants showed a significant decrease of 40% in SD at Ψo = −2.6 MPa (Figure 3(a)).
Similarly to SD, epidermal cell density (ECD) in the abaxial surface showed a significant increase in Na2SO4 treated plants at moderate to high salinity. In the adaxial surface an increase was observed only at −1.9 MPa for ECD and SD. In NaCl and NaCl + Na2SO4 treated plants a significant decrease of ECD and SD on both leaf sides was observed (at −2.6 MPa in the abaxial surface and at −1.9 and −2.6 MPa in the adaxial surface) (Figure 3(b)). For stomatal index (SI) a significant increase was found in Na2SO4 treated plants at −1 MPa for the adaxial surface, in relation to controls and NaCl treated plants. At moderate salinity, NaCl + Na2SO4 treated plants showed an increase of SI also in the adaxial surface in relation to control plants only (Figure 3(c)).
3.3. Effects of Salinity on Stomatal Characteristics: Length, Width and Area
As shown in Figure 4, salt treatments significantly affected stomata size, showing variations in area, length
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Effects of NaCl, Na2SO4, and their iso-osmotic mixture on SD (a) ECD (b) and SI (c) in P. strombulifera plants. Means values (±S.E.) followed by different letters above bars are significantly different at P < 0.05 (n = 15).
 (a)
(a) (b)
(b) (c)
(c)
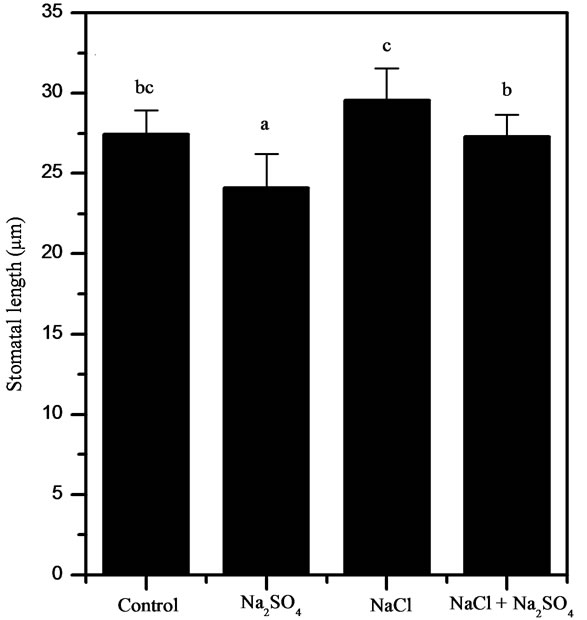
Figure 4. Stomatal measurements on 48 days-old P. strombulifera plants (Ψo = −2.6 MPa). Length (a), width (b) and area (c). Means values (±S.E.) followed by different letters above bars are significantly different at P < 0.05 (n = 15).
and width of guard cells in P. strombulifera seedlings.
In Na2SO4 treated plants at Ψo = −1.9 MPa there was a significant decrease in the guard cells length in relation to controls and others saline treatments (Figure 4(a)), whereas NaCl and NaCl + Na2SO4 treated plants showed a significant increase in the guard cell width (Figure 4(b)). The stomatal area was the trait most influenced by salt stress showing a significant decrease in Na2SO4 treated plants (25%). In contrast, NaCl treatment significantly increased (12%) the area of the stomata in these plants (Figure 4(c)).
3.4. Transpiration
Up to 12 h after the salt pulse, none of the salt treatments affected transpiration but at 24 h, water loss sharply increased in all treatments. At 48 days of culture, the Na2SO4-treated plants showed the highest transpiration exceeding the level in the control plants. Transpiration was the lowest in NaCl − and NaCl + Na2SO4 treated plants, and no significant differences were observed between these treatments (Figure 5).
4. Discussion
Upon prolonged stress exposure, plants respond with structural changes, including morphological adaptations such as modifications of the root-to-shoot ratio and modifications of stomatal density and distribution, which have both been proven to be critical under salinity [34]. The potential surface available for regulated gas exchange between plants and the atmosphere is set by stomatal number and distribution in the aerial epidermis [23]. Stomatal abundance in different plant surfaces and

Figure 5. Effects of NaCl, Na2SO4, and their iso-osmotic mixture on transpiration in P. strombulifera plants. Means values (±S.E.) followed by different letters above bars are significantly different at P < 0.05 (n = 5).
environments is regulated, resulting in variable stomatal numbers and distribution patterns in mature organs [35,36]. In this study, we observed that P. strombulifera has amphistomatic leaves [37], with more abundant stomata in the adaxial surface. The amphistomatous nature of these leaves might mean that they have the capacity to open and close their stomata on both sides independently with transpiration rates being more sensitive to changes in stomatal aperture on the abaxial surface, as observed in maize [38].
Prosopis strombulifera Na2SO4-treated plants showed an increase in SD and ECD (with smaller stomata), but no variation in SI, whereas in NaCl and NaCl + Na2SO4 treated plants a decrease in these variables was observed. High number of smaller stomata is a xerophytic trait which maybe related to a greater photosynthetic demand and an increased need of stomatal conductance regulation to balance between photosynthetic rate and transpiration. Differences in the stomatal area may be due to the size of guard cells, on the one hand, or to differences in the pore opening, on the other hand, which modify the specific area occupied by the stomata. Additionally, the presence of a larger number of small stomata may improve functionality and coordination among different stomata [39, 40]. This coordinated behavior could induce “stomatal patchiness” as result of hydraulic interactions that produce a fast patchy stomatal closure [41]. In coincidence, on Thellungiella halophylla leaves the SD is twice that of Arabidopsis leaves although the SI is nearly the same, which was proposed to allow more efficient distribution of CO2 to photosynthetic mesophyll cells at low stomatal apertures [42]. On the other hand, we have previously observed that leaves of NaCl-treated plants showed great osmotic adjustment capacity. Such capacity was dramatically lower when sulfate anion was present in the medium (Na2SO4 or bisaline-treated plants) [43]. Na2SO4-treated plants had a strongly negative osmotic and water potentials that cause water imbalance, correlated with the reduction in individual and total leaf area at Ψo = −1.9 or −2.6 MPa [11]. It is traditionally assumed that as leaf expansion growth is reduced by salinity [44], cells become smaller in size, and this results in a larger number of cells per unit surface area (i.e. increased cell density). It is therefore possible that all these settings in the epidermal morphology are closely related to water use and photosynthetic efficiency in salinized seedlings of P. strombulifera. Notably, when chloride and sulfate are together in the medium the response is similar to that in NaCl-treated plants or intermediate, suggesting that both anions interact at membrane level in an antagonistic manner.
Halophytes typically exhibit reduced transpiration rates when compared with glycophytes. Decreases in stomatal opening in halophytes prevent excessive water loss and reduces ion movements by the transpiration stream [45].
In this study, P. strombulifera Na2SO4-treated plants showed the highest transpiration at moderate and high salinity. Additionally, in the presence of this salt, the highest content of ABA was registered [46]. A possible explanation is that despite these high ABA levels there is no inhibition of stomatal opening resulting in increased water loss, growth inhibition, and acceleration of sensecence processes [11]. Earns et al. [47] suggested that sulfate ion has an interactive effect on ABA, resulting in greater reduction of transpiration rate in maize and of stomatal opening in Vicia faba compared to ABA alone. The antitranspiratory effect of ABA reaching stomata at early stage is enhanced by increased content of sulfate ion which is also transported within the xylem, and effectively down-regulates transpiration in these systems. In contrast, in P. strombulifera, sulfate ion and ABA content were much higher in leaves and roots of Na2SO4-treated plants and had no effect on closing stomata. Sulfate ion accumulation and the stress imposed by the presence of this anion in tissues seemed to be interfering at some point with the ABA signaling pathway blocking the activity of ABA. Thus, stomata remained open and high transpiration values were recorded [46]. In contrast, NaCl treatment significantly decreased the SD and ECD, and increased the area of the stomata. Decreases in stomatal density with increasing salinity have also been found in several highly salt tolerant halophyte species such as Kochia prostrata [48], Suaeda maritima [49], Distichlis spicata [50], Atriplex halimus and Medicago arborea [51], and Ael ropus lagopodies and Lasiurus scindicus [52]. Accordingly, [53] studied six clones of Jojoba under salinity conditions in northern Chile, and reported that the most salt resistant of the clones had the largest stomata and much lower trichome and SD than the other clones. In the succulent halophyte Kandelia candel conditions of high salinity (500 mM NaCl) caused lower stomatal number per leaf and reduced leaf thickness [54]. Reduction in stomatal density is a highly conserved response employed not only by halophytes, but also by crop species as a part of their adaptation to salinity [55]. The qualitative model put forward in [56] suggests that a reduction in SD will be a critical determinant for high water use efficiency (WUE) under saline conditions, according with other authors [57]. Similarly, [58] demonstrated that reduced stomatal density, high ascorbate level and polyphenol oxidase (PPO) activity coordinately contribute to improve basil adaptation and WUE in saline environment. The constitutively reduced stomatal density was associated with a “delayed” accumulation of stress molecules (and growth inhibiting signals) such as abscisic acid (ABA) and proline, in the more tolerant Genovese.
These results are consistent with our previous ones which showed that NaCl is not deleterious for this species being well tolerated up to concentrations as high as 700 Mm (−2.6 MPa). NaCl-treated plants grew well, their aspect was healthy without showing toxicity [11]; thus, they may not need an extra ABA accumulation for adaptive mechanisms for plant survival such as SD and ECD modifications to improve WUE and photosynthesis. On the contrary, in Na2SO4-treated plants, besides the scarce  accumulation in tissues, were sufficient to cause metabolic disorders manifested as chlorosis, necrosis and leaf abscission [11]. Leaf development, particularly, lead to structural modifications in leaf size and micro-morphology of leaf cells in an attempt to overcome the highly stressing situation generated by sulphate. Thus, in addition to induced structural alterations in cells and tissues, with consequent changes in growth patterns at various levels of organization [11], Na2SO4 treatment causes the inability of the plant to build a halophytic response as it does with NaCl. Indeed, an increased number of smaller stomata (which are not responsive to ABA), increased accumulation of stress molecules and growth inhibiting signals [44] plus a high transpiration rate, constitute a lethal situation for Na2SO4 treated plants.
accumulation in tissues, were sufficient to cause metabolic disorders manifested as chlorosis, necrosis and leaf abscission [11]. Leaf development, particularly, lead to structural modifications in leaf size and micro-morphology of leaf cells in an attempt to overcome the highly stressing situation generated by sulphate. Thus, in addition to induced structural alterations in cells and tissues, with consequent changes in growth patterns at various levels of organization [11], Na2SO4 treatment causes the inability of the plant to build a halophytic response as it does with NaCl. Indeed, an increased number of smaller stomata (which are not responsive to ABA), increased accumulation of stress molecules and growth inhibiting signals [44] plus a high transpiration rate, constitute a lethal situation for Na2SO4 treated plants.
The deleterious effects of  on leaf development, and on root and shoot elongation, are presumably a consequence of several metabolic reactions, e.g. sulphide formation in the process of sulphate assimilation in the chloroplast, wherein sulphite reductase catalyses the reduction of sulphite to sulphide using reduced ferredoxin as electron donor [59]. Free sulphide can be incorporated into L-cysteine through cysteine synthase, which is the most efficient way to keep its concentration low in order to avoid inhibitory effects. If all free sulphide is not consumed by this assimilatory step, it could be released to the environment or could bind to cytochromes, thereby inhibiting mitochondrial respiration [60]. The mechanism of the specific
on leaf development, and on root and shoot elongation, are presumably a consequence of several metabolic reactions, e.g. sulphide formation in the process of sulphate assimilation in the chloroplast, wherein sulphite reductase catalyses the reduction of sulphite to sulphide using reduced ferredoxin as electron donor [59]. Free sulphide can be incorporated into L-cysteine through cysteine synthase, which is the most efficient way to keep its concentration low in order to avoid inhibitory effects. If all free sulphide is not consumed by this assimilatory step, it could be released to the environment or could bind to cytochromes, thereby inhibiting mitochondrial respiration [60]. The mechanism of the specific  effect in this species is currently under study in our laboratory.
effect in this species is currently under study in our laboratory.
5. Conclusion
In conclusion, this study demonstrates that plant growth’s responses to salinity may be completely different depending on the anion associated with sodium in the soil solution, reinforcing the need of performing salinity experiments taking into account a more realistic chemical composition of the solutions to mimic what occurs in the flied. Also, the present results contribute to better understanding the stomatal development as a fundamental aspect of morphophysiological responses of the halophyte P. strombulifera to increasing salinization with different sodium salts. Recently, reduced SD in halophytes was highlighted as a key feature that has never been manipulated by breeders [55]. This opens up novel and previously unexplored possibilities for improving salinity tolerance in crops.
6. Acknowledgements
This study was supported with funds from CONICET, ANPCYT, SECYT-Universidad Nacional de Río Cuarto and Ministerio de Ciencia y Tecnología de la Provincia de Córdoba (R No 1210/2007), Argentina, to V. Luna, and a fellowship from CONICET to M. Reginato.
NOTES
#Corresponding author.