Combined Effects of Temperature and Nutrient Availability on Growth and Phlorotannin Concentration of the Brown Alga Sargassum patens (Fucales; Phaeophyceae) ()
1. Introduction
Global warming is predicted to change plant-herbivore interactions in terrestrial environments. Result of metaanalysis showed that elevated temperature had a favorable effect on the performance of insect herbivores and an inconsistent effect on the secondary chemistry of plants [1]. Responses of defensive secondary compounds appear to depend on the plant species and the chemical type. For example, concentrations of nonstructural carbohydrates and phenolics decreased under elevated temperature, whereas terpene concentration increased at high temperature [1,2].
Warming is also predicted to affect marine plant-herbivore interactions. For example, O’Connor [3] reported that an increase in temperature from 23˚C to 29˚C did not affect the palatability of the brown alga Sargassum filipendula but did increase its rate of consumption by the amphipod Ampithoe longimana. In contrast, Poore et al. [4] found that a temperature increase from 23˚C to 26˚C increased the palatability of the brown algae Sargassum linearifolium but had no effect on the feeding rates of the amphipod Peraphithoe parmerong. Sudatti et al. [5] reported that the concentration of defensive compounds (elatol) in the red alga Laurencia dendroidea increased with increasing temperature between 15˚C and 25˚C but decreased at 30˚C (also see reference therein). However, little information is available about the effects of temperature on marine macroalgal secondary chemistry and how these effects may impact plant-herbivore interactions.
Large brown algae that belong to the orders Laminariales (kelp) and Fucales (fucoid), including Sargassum spp., are the dominant taxa on intertidal and subtidal rocky shores in temperate and subarctic regions of the world [6,7] and play an ecologically important role by providing habitats and nurseries to a wide range of organisms [8,9]. These algae can become physiologically stressed due to the combined effects of above-average temperatures and nutrient-poor conditions during summer [7,10], and they frequently experience intensive grazing by herbivorous animals such as molluscs, sea urchins, and fishes [11]. Brown algae contain unique compounds called phlorotannins, which are known to have secondary roles as chemical defenses (e.g., herbivore deterrents, digestion inhibitors, antibacterial agents, and UV screens) and primary roles in constructing and strengthening the cell walls [12]. The concentrations of phlorotannins are known to be affected by abiotic and biotic factors such as nutrient availability, UV-B radiation, and grazing [12], but little is known about the effect of temperature. Poore et al. [4] reported that elevated temperature (from 23˚C to 26˚C) had no effect on the phlorotannin concentration of S. linearifolium. However, temperature might affect phlorotannin concentration indirectly via changing the growth rate of brown algae, as phlorotannin concentrations reportedly decrease with increasing growth rate [13-16]. Hence, to estimate the direct or indirect effect of temperature on phlorotannin concentration, the effects of minimal and optimal growth temperature on phlorotannin concentration need to be tested. Moreover, the temperature effect might be influenced by nutrient availability, as nutrient limitation might increase resource allocation to defensive secondary metabolites [13]. In addition, response of phlorotannin concentration to abiotic factor might differ between upper and lower parts of shoots in Sarggassum spp., as reported by Hay et al. [17].
In southern Japan, decline of dominant kelp Ecklonia spp. and distributional shifts of dominant fucoid Sargassum spp. were observed in response to recent warming during the last few decades [18]. These algae are also currently experiencing intensive herbivory by the endemic fish species, because warming allows these fish species to graze actively for longer periods from summer to winter [19]. However, there was no study to test the possibility that elevated temperature might increase the palatability of Japanese Sargassum spp.
Sargassum patens is one of the dominant species on subtidal rocky shores around Japan [20], including Hong Kong [21]. This species has a perennial discoid holdfast, some perennial short stipes, and many annual shoots that are produced during summer, grow from autumn to spring, and decay the next summer after maturation [21,22]. Physiological studies of Sargassum spp. have been conducted via culture experiment using shoots excised from the holdfast or stipe [23-25], but this procedure can be problematic because differences in the ratio of axes, leaves, and vesicles within a shoot affect physiological parameters such as photosynthetic rate [26]. S. patens has flat axes that are indistinguishable from their flat leaves [20] (see Figure 1), thus physiological differences between axes and leaves might be relatively small. Hence, the new short shoots excised from this species during summer are suitable for culture experiments in flasks to test the effect of various abiotic factors, including warm summer temperature. Moreover, optimal and critical temperatures for survive of this species already have been estimated to be 20˚C - 25˚C and 31˚C, respectively, based on culture experiments using excised shoots [27].
In the present study, we conducted a culture experiment using excised shoots of S. patens to test the combined effect of temperature and nutrient availability on growth and phlorotannnin concentration. Because S. patens experiences temperatures ranging from ca. 10˚C (winter temperature at its northern limit) to 30˚C (summer temperature at its southern limit) within its distributional range and its optimal growth temperature is 20˚C - 25˚C [27], excised shoots from this species were cultured at 10˚C, 20˚C, 30˚C in both nutrient-enriched medium and non-enriched seawater. Relative growth rate (RGR) and phlorotannin concentration of S. patens were measured in each treatment. We addressed the following questions: 1) Is the effect of temperature on phlorotannin concentration significant? 2) Does temperature have an indirect effect on phlorotannin concentration by changing the growth rate? 3) Does the effect of temperature differ between the upper and lower parts of the shoots? 4) Does nutrient availability influence the effect of temperature on phlorotannin concentration?
2. Materials and Methods
2.1. Culture Experiment
S. patens specimens with several short shoots were collected in July 2012 from a sheltered site along the coast at Unosaki (39˚51'20'' N, 139˚48'56'' E), which is located on the Oga Peninsula, Akita Prefecture, northern Japan. These specimens were transported to our laboratory in insulated cool boxes, and apical shoots without vesicles

Figure 1. Shoot morphology of the brown alga S. patens.
were excised from each specimen (Figure 1). To reduce the negative effects of excision, these shoots were placed in flasks containing 500 ml of enriched 25% PESI medium [28], which was made using filtered seawater pumped from Unosaki. These shoots were maintained in incubators with aeration at the optimal growth temperature (20˚C) for ca. 24 h until the start of the experiment. Light was provided by eight 40 W cool white fluorescent tubes in the incubators. Photon flux density was set at 70 μmol photons m−2∙s−1. Photoperiod was set at 12 h L (light):12 h D (dark).
To evaluate the combined effect of temperature and nutrient availability on growth rate and phlorotannin concentration, shoots were cultured at 10˚C, 20˚C, 30˚C in both nutrient-enriched (25% PESI) and non-enriched medium made from filtered seawater from Unosaki (i.e., a total of six treatments). Each treatment was replicated by eight times using shoots excised from eight different individuals because physiological response might differ among individuals [29]. These shoots were maintained at a photon flux density of 180 μmol photons m−2∙s−1 (saturation light level for photosynthesis of this species [30]) and a photoperiod of the 12 h L:12 h D for 9 d. The culture medium in each flask was changed every 3 days. The concentrations of ammonia, nitrate, nitrite, and phosphate in both nutrient-enriched and non-enriched medium were measured at the start of the experiment using an autoanalyzer (QuAAtro 2-HR; BLTEC, Japan) with replication of 5 times.
2.2. Measurement of Relative Growth Rate
Wet weights of the shoots prior to the experiment (initial) and at the end (final) were measured using an electrical balance (0.1 mg accuracy) after removal of excess moisture by blotting on paper towels. The initial wet weight of shoots was 1.416 ± 0.498 g. These shoots were cut into halves (upper and lower parts), and 0.2 g (wet weight) aliquots of the tissues were excised from each part for measurement of phlorotannin concentration. The remaining tissues from each part were weighed before and after drying for 72 h at 80˚C in a convection oven. The ratios of dry weight to wet weight for each part of the shoots were used to convert the final wet weight into dry weight. To convert the initial wet weight into dry weight, the ratios of dry weight to wet weight of another eight shoots measured at the start of the experiment were used. The RGRs (% d−1) were calculated as 100 × ln (final dry weight/initial dry weight)/9 d.
2.3. Measurement of Phlorotannin Concentration
Each 0.2 g aliquot of tissue from each part of the shoot was immediately placed in a 15 mL conical tube with 4 mL of 80% aqueous methanol at 4˚C in the dark for 10 d. The phlorotannin concentrations of each sample was measured using the Folin-Ciocalteu method, which provides a more consistent result and is less prone to interference by non-phenolic compounds than other colorimetric methods such as the Folin-Denis and Price-Butler methods [31], according to Kamiya et al. [32]. After incubation for 10 d, the tissues were ground with 6 mL of 80% aqueous methanol using a mortar and pestle. This solution was centrifuged at 3500 rpm at 4˚C for 15 min, and then the volume of the supernatant was measured (0.05 mL accuracy). One milliliter of the extract was placed in a microtube and centrifuged at 14,000 rpm at 4˚C for 5 min. Next, 50 μL from each extract or 80% aqueous methanol (as a reference) were added to 1 mL of distilled water and 1 mL of 40% Folin-Ciocalteu reagent (F9252, Sigma-Aldrich, St Louis, MO, USA) in an 8 mL glass tube. After 5 min incubation at room temperature, 1 mL of 2N Na2CO3 was added, followed by incubation in a 50˚C water bath for 30 min. The absorbance of each solution was then measured at 765 nm using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). Anhydrous phloroglucinol (1,3,5-trihydroxybenzene, 322-56502, Wako, Osaka, Japan) was used as the standard. The final phlorotannin concentraions were expressed as a percentage of the dry mass.
2.4. Statistical Analysis
Significance of effects of temperature and nutrient availability on RGR of S. patens shoots was analyzed by twoway ANOVA and Tukey’s multiple comparison test after logarithmic transformation because some data were not normally distributed (Shapiro-Wilk test, P < 0.05) and did not show homogeneous variances (Levene test, P < 0.05). Significance of effects of temperature, nutrient availability, and parts of shoot (upper or lower part) on phlorotannin concentration of S. patens was analyzed by three-way ANOVA and Tukey’s multiple comparison test without data transformation because some data were normally distributed (Shapiro-Wilk test, P > 0.05) and show homogeneous variances (Levene test, P > 0.05). Correlations between RGR and phlorotannin concentration were analyzed using the Pearson’s test. These analyses were performed using SPSS Statistics 20.0 (IBM, Armonk, NY, USA).
3. Results
The concentrations of ammonia, nitrate, nitrite, and phosphate in the nutrient-enriched medium were 0.122 ± 0.008, 2.007 ± 0.083, <0.005, and 0.034 ± 0.002 mg/l, respectively, whereas those in the non-enriched medium were 0.026 ± 0.003, 0.009 ± 0.002, <0.005, and <0.005 mg/l, respectively.
Figure 2 shows the RGRs of S. patens shoots cultured in the six treatments. Results of two-way ANOVA revealed a significant effect of temperature on RGR but no significant effect of nutrient enrichment and no significant interaction between temperature and nutrient enrichment on RGR (Table 1). Results of Tukey’s multiple comparison test showed that RGRs of the shoots cultured at 10˚C were significantly lower than those of the shoots cultured at 20˚C and 30˚C in both enriched and non-enriched treatments.
Figure 3 shows the phlorotannin concentrations of the upper and lower parts of shoots cultured in the six treatments. Results of three-way ANOVA showed that there was a significant effect of nutrient enrichment and a significant interaction between temperature and part of the shoots on the phlorotannin concentration. However, significant effects of temperature and part of the shoot on the phlorotannin concentration were not observed (Table 1). Results of Tukey’s multiple comparison test showed that the phlorotannin concentration of the upper part of the shoots cultured at 10˚C in non-enriched medium was significant higher than those of the lower part of the shoots cultured under the same condition and of the upper part of the shoots cultured at 10˚C in enriched medium.
Figure 4 illustrates the relationships between RGR and phlorotannin concentration of the upper and lower parts of the shoots cultured in enriched and non-enriched medium. As there was no significant effect of temperature on phlorotannnin concentration, shoots cultured at all three temperatures were regarded as one group in this analysis. Significant correlations between RGR and phlorotannin concentration were observed in the upper part of the shoot in both the enriched and non-enriched treatments, but there was no significant correlation in the low part of the shoot in either medium. Whereas a posi-

Figure 2. The RGRs (relative growth rates) of S. patens shoots cultured in nutrient-enriched and non-enriched medium. White, grey, and black bar indicate the RGRs at 10˚C, 20˚C, and 30˚C, respectively. Different small letters indicate statistical significances (p < 0.05) among different temperatures. Mean ± SD; n = 8 shoots.

Figure 3. The phlorotannin concentrations of the upper and lower parts of S. patens shoots cultured in both nutrientenriched and non-enriched medium. White, grey, and black bar indicate the phlorotannin concentrations at 10˚C, 20˚C, and 30˚C, respectively. Different small letters indicate statistical significances (p < 0.05) among different treatments. Mean ± SD; n = 8 shoots.
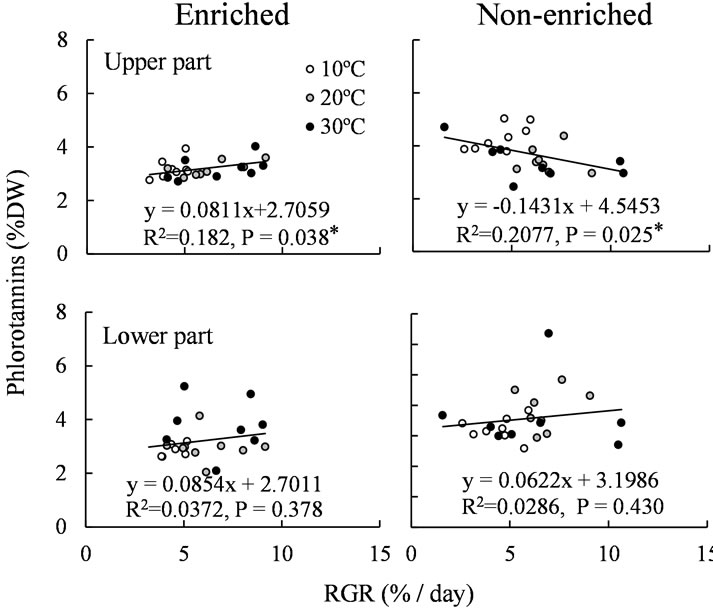
Figure 4. The relationships between RGR and phlorotannin concentration of the upper and lower parts of S. patens shoots cultured in nutrient-enriched and non-enriched medium. White, grey, and black points indicate the phlorotannin concentrations at 10˚C, 20˚C, and 30˚C, respectively.
tive correlation was observed in the upper part of the shoot cultured in the enriched medium, a negative correlation was observed in the same part of the shoot cultured in the non-enriched medium.
4. Discussion
Poore et al. [4] reported that an increase in temperature from 23˚C to 26˚C had no effect on the phlorotannin concentration of S. linearifolium in their 14 d study. Similarly, we found no significant effect of temperature within the range of 10˚C - 30˚C on phlorotannin concentration of S. patens in our present 9 d study. These results indicate that direct effects of temperature on phlorotannin concentration of Sargassum spp. are relatively small or cannot be detected in short-term experiments. However,
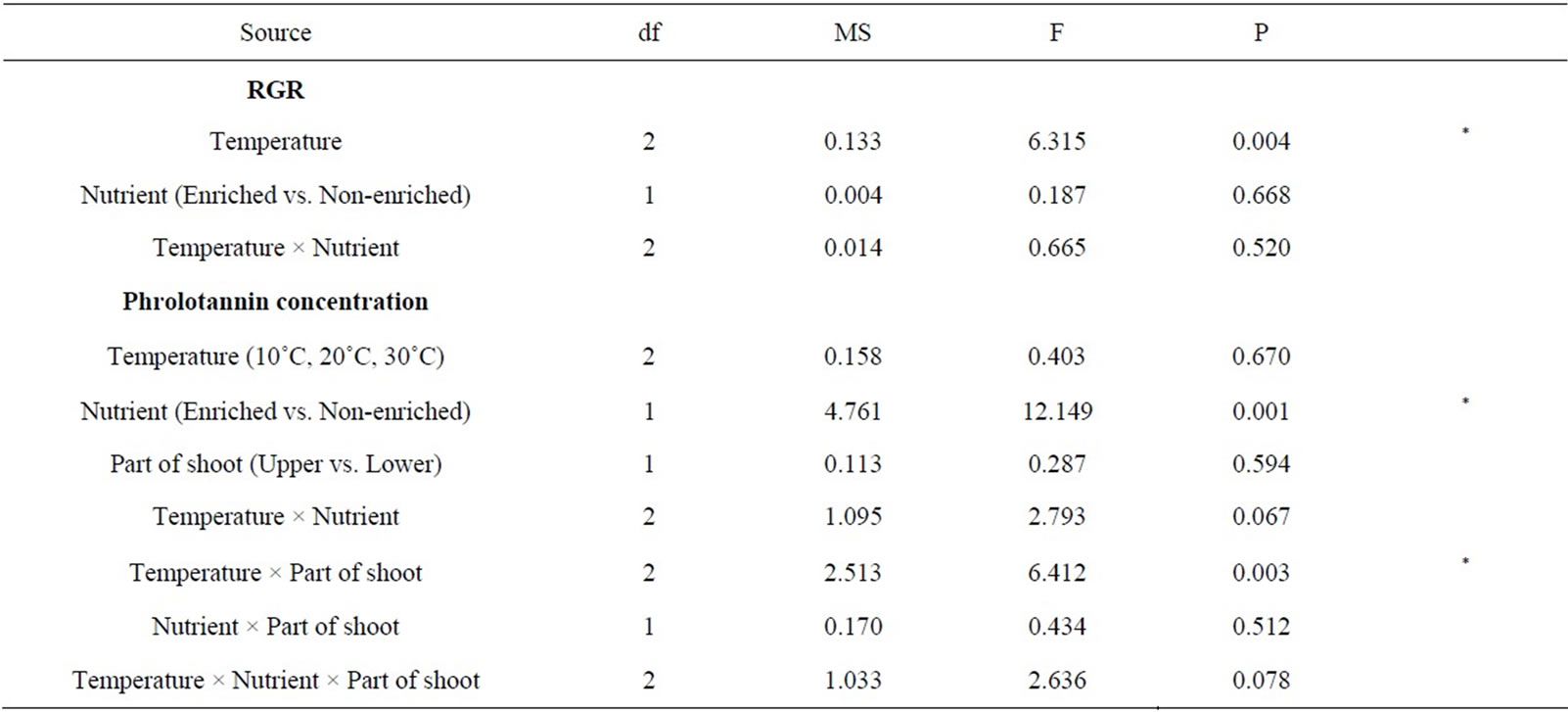
Table 1. Results of ANOVA on the effect of temperature, nutrient, and part of shoot on RGR and phlorotannin concentration of S. patens shoots.
we found that the RGR of S. patens shoots was significantly affected by temperature and that the phlorotannin concentration of the upper part of the shoots decreased with increasing RGR in non-enriched medium. These results suggest that temperature indirectly affects phlorotannin concentration of S. patens by changing the growth rate, but only in the upper part of the shoots and in the ambient nutrient condition.
The inverse relationship between growth rate and phlorotannin concentration has been reported previously [13-16]. Arnold and Targett [16] proposed that the reactive, extractable, and therefore measurable phlorotannins sequestered in the physodes (membrane-bound vesicles in the cytoplasm, which contains the high concentration of phlorotannins in the cell) serve as secondary compounds and subsequently transition to a primary role in constructing and strengthening the cell wall. This model explains that measurable phlorotannin levels can drop when the requirement for cell wall construction (i.e. growth) is high and can increase in slow-growing tissue in which rates of phlorotannin synthesis outpace rates of decomposition and incorporation of phlorotannins into cell walls [12,16]. In the present study, a significant correlation between RGR and phlorotannin concentration was observed in the upper part of S. patens shoots but not in the lower part of shoots, and was negative in the nonenriched medium but slightly positive in the enriched medium. These results can be explained by the above model, as Sargassum has meristems (fast-growing tissue) in the upper part of the shoots, and nutrient enrichment usually enhances growth of Sargassum spp. [23-25]. Although a significant effect of nutrient availability on growth rates was not detected in the present study, lower values of RGR (1.6% - 3.2%) were frequently observed in the non-enriched medium but not in the enriched medium. In addition, phlorotannin concentration of the upper part of the shoots cultured at 10˚C in non-enriched medium was significantly higher than that in shoots cultured at the same temperature in the enriched medium. Therefore, the negative correlation between RGR and phlorotannin concentration of the upper part of S. patens shoots in non-enriched medium seems to be a result of the increased concentration of phlorotannins under slow growth rate at 10˚C rather than decreased concentration under fast growth rate at 30˚C.
Results of the present study showed that increasing temperature indirectly affects the phlorotannin concentration of S. patens shoots by increasing the growth rate. As the phlorotannin concentration of the upper part of the shoots slightly decreased with increasing growth rate in the non-enriched medium, S. patens shoots seem to become palatable to herbivorous fishes, which mainly graze upper part of shoots, under warm temperature and nutrient-poor conditions during summer in the fields. In fact, Sargassum spp. are currently experiencing intensive herbivory by endemic fish species such as the rabitfish Siganus fuscescens in southern Japan [19], where the seawater temperature has increased during the last few decades [18]. However, the temperature effect on secondary chemistry of brown algae, including Sargassum spp., is poorly understood, which makes it difficult to explain the phenomena occurring in the algae fields. Although many studies have classified phlorotannin concentrations at the end of experiments as “phlorotannin levels”, the turnover rate of phlorotannins [33] should be considered to better understand the effect of abiotic factors on phlorotannin levels. Temperature might also affect the traits of other secondary chemicals [4]. Further studies are needed to test the effects of temperature on marine plant secondary chemistry and to determine how these effects may impact plant-herbivore interactions.
5. Acknowledgements
We thank Prof. M. Kamiya of Fukui Prefectural University for teaching the determination methods of phlorotannin concentration. We also thank Mr. A. Nakamura, Mr. Y. Shirohata, and Mr. J. Yamada of Akita Prefectural Fisheries Promotion Center for their help with sample collection. This work was supported by JSPS KAKENHI Grant Number 24780177.