Adherence of Community Pharmacies in Riyadh, Saudi Arabia, to Optimal Conditions for Keeping and Selling Good-Quality Medicines ()
1. Introduction
The quality of medicine is a global issue due to the ever increasing prevalence of counterfeit medicines worldwide [1,2]. Substandard medicines produced by legitimate manufacturers represent another threat as they may not contain the correct amount of active ingredients or may have manufacturing defects that alter their efficacy or make them dangerous for use [3,4]. On the other hand, even good quality medicines could be risky if they degrade due to poor storage or distribution conditions [5, 6].
This study is based on the results of a previous study which is submitted for publication [7]. That study investigated the quality of amoxicillin capsules and tablets sold in community pharmacies in Riyadh, Saudi Arabia. It was conducted during a very hot season and amoxicillin was selected as an indicator of the quality of medicines in those pharmacies. The study reported substandard amoxicillin products in 13% of the randomly selected pharmacies. Although all samples were found to be authentic and the amount of the active ingredient was not dramatically low, it was below the pharmacopoeial limits with the lowest amount being 80.7%. Manufacturing defects could not be ruled out but it was also suggested that degradation may have occurred to some samples due to poor storage or distribution conditions.
That was supported by the finding that certain samples passed the quality test while other samples from the same batch, but purchased from other pharmacies, failed it.
Most medicines that can be kept on shelves must not be exposed to temperatures exceeding 25˚C. Excessive heat plays a major role in the degradation of various medicines [8,9]. Similarly, temperature-controlled medicines must be handled carefully to avoid decomposition or degradation caused by excessive heat [10].
The operational regulations of the system for private pharmaceutical facilities and products in Saudi Arabia contain several requirements for good storage and distribution of medicines [11]. According to this system, community pharmacies must have good air-conditioning systems that keep the temperature at ≤25˚C and a thermometer for monitoring the temperature. Pharmacies must also have refrigerators with thermometers and medicines must be kept away from sunlight. Floors and paints must be of materials that can be easily washed and cleaned. In addition, drug storage facilities must be equipped with automatic temperature recording systems that keep records for at least one year.
However, the criteria and quality of inspection and monitoring of the community pharmacies by the regulatory authorities may not be efficient. For example, one can easily buy prescription-only medicines, such as antibiotics, without a prescription despite the strict regulations [12].
Our current study explored the extent to which community pharmacies in Riyadh complied with the local regulations for keeping medicines until they are sold. We also explored the opinions of community pharmacists about the quality of medicines and tested their knowledge about the regulations.
2. Materials and Methods
2.1. Ethical Issues
This study was approved by the Ethics Committee of Kanazawa University and the Saudi Food and Drug Authority (SFDA), and was conducted between November 2 and November 23, 2012.
2.2. Selection of Pharmacies
A list of all registered community pharmacies in Riyadh was obtained from the Saudi Ministry of Health (MOH) in August 2012 (1531 pharmacies). The calculated sample size of pharmacies was 181 according to the following formula:

where n is the required sample size; z is the reliability coefficient (equals 1.96% at 95% confidence level); N is the population size; p is the probability of favorable outcome (set as 0.5); and ξ is set as 0.2 for the purpose of this study.
After all pharmacies were coded, the list was scrambled and 181 + 45 pharmacies were randomly selected from the list with MS Excel 2010 (Microsoft Co., USA). The additional 45 pharmacies were used as a reserve for an estimated dropout rate of 25% that may occur when a pharmacy is closed on the second visit, the pharmacy is out of business, or the pharmacy refuses to cooperate in the survey.
2.3. Study Materials
The survey was conducted using a structured interview with the pharmacist in charge in each pharmacy, using 3 forms that were filled in by the interviewers. The first form was a questionnaire while the second and third forms were used for inspection and observation purposes, respectively. Validity of the method was assessed on a sample of 33 pharmacies, not included in the study sample, and the Pearson correlation coefficient for internal reliability was 0.82.
2.4. The Interviewers
Thirty-four, fourth-year pharmacy students from King Saud University in Riyadh conducted the interviews after having attended a workshop followed by field training for a period of one week accompanied by the chief investigator. In-field follow-up and necessary support were provided by the chief investigator and one of the co-investigators.
3. Results
The final sample included 139 chain and 42 independent pharmacies, where a chain pharmacy belongs to a group of >3 pharmacies [13]. Twenty-one pharmacies were replaced because they were either closed on the second visit, out of business, or non-cooperative. The pharmacists in charge were all non-Saudi males and the summary of their background characteristics is shown in Table 1.
About 15% of the pharmacists said that they had not been informed about the system of community pharmacy practice in Saudi Arabia. Surprisingly, a significant percentage of pharmacists who knew the system gave wrong answers to simple questions related to the system. Table 2 summarizes the pharmacists’ answers to such questions.
All pharmacists reported that they always receive the supply of medicines either directly from an official distributor or from a central store that belongs to the owner, or both. However, one independent pharmacy reported purchasing some medicines from a subagent. Nearly 56%
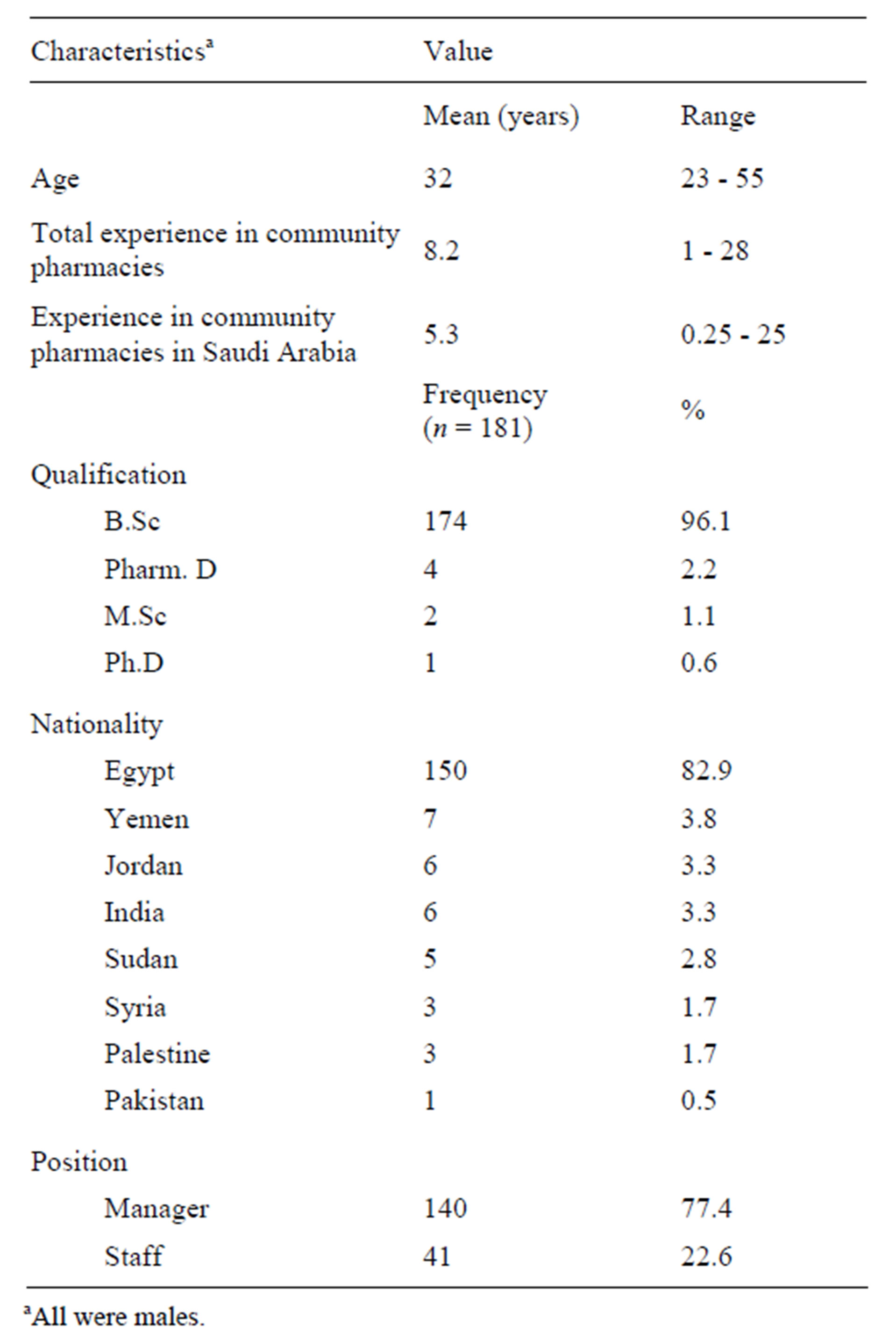
Table 1. Background characteristics of pharmacists.
aAll were males.
of the pharmacists reported that they sometimes rejected some supplies of medicines because the temperature of the shipments may have exceeded 25˚C or because the packaging of the medicines was not intact upon receipt. When asked about the official distributors’ facilities, 16% of the pharmacists said that the distributors’ stores were substandard and 22% believed that the vehicles that were delivering the supply to the pharmacies were not suitable. In addition, 8% reported that they found some counterfeit products in their pharmacies and 46% reported that they had sometimes been contacted by illegal sellers offering known medicine brands at low prices. A significant percentage of the pharmacists reported that they found physicochemical changes in some of the medicines in their pharmacies: 16% noticed discoloration of some liquid medicines while 18.8% noticed wetting or solidification of powdered medicines. Sixty-seven percent of the pharmacists reported that they received complaints from their clients about subtherapeutic effects of some medicines. Only 64% said that they received memos from the drug regulatory authorities warning them of certain counterfeit or substandard products. Table 3 summarizes the pharmacists’ opinions about the compliance of their pharmacies as well as their drug distributors with the local regulations.
In all pharmacies, excess medicines were stored in a small room inside each pharmacy. The inspection revealed serious problems regarding the temperature control in the pharmacies and in their refrigerators. Generally, the degree of cleanliness and neatness of pharmacies was considered acceptable since no serious breach was found. A summary of the recordings of inspections and observations made in all pharmacies is presented in Table 4.
Various scoring systems were used in different studies concerned with the quality of pharmacies for a variety of statistical analyses [14,15]. In our study, however, when
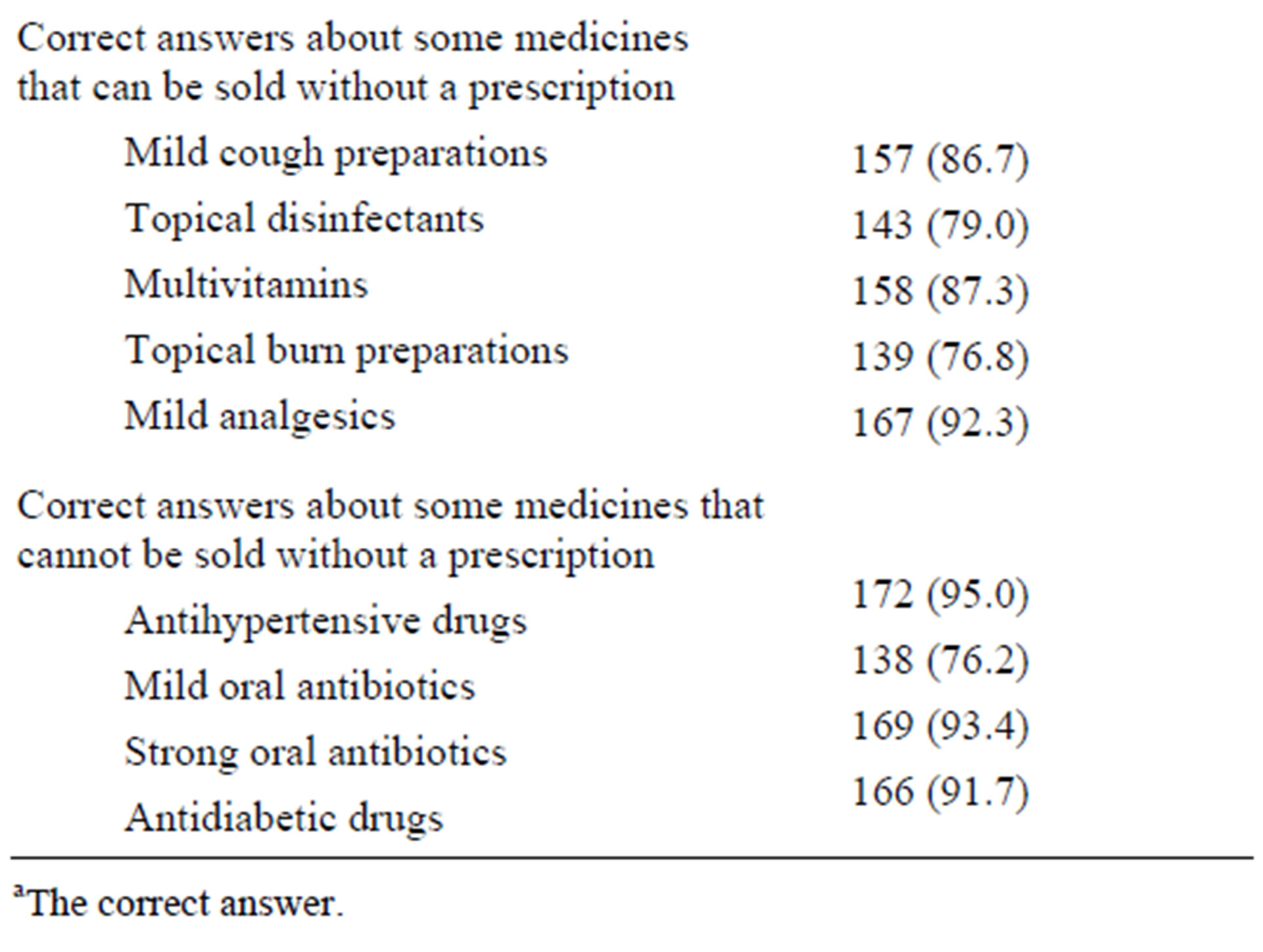

Table 2. Pharmacist knowledge about the local regulations of community pharmacy practice.
aThe correct answer.


Table 3. Pharmacists’ opinions on the adherence of their pharmacies and distributors to the regulations.
aInfrequent and lasted for 15 - 180 minutes.

Table 4. Pharmacy inspection results and observations.
aReadings as high as 30˚C were observed in some pharmacies. bReadings as low as ‒10˚C and as high as 20˚C were observed in some pharmacies. cFree of dust, dirt, insects, or spider webs.
scores were added to key observations, no significant differences were found between the means of various independent factors, for example, chain vs. independent pharmacies, managers vs. staff, pharmacist informed about the regulations in Saudi Arabia vs. those not informed, and pharmacists aware of the continuing education requirements vs. those who were unaware. The analysis of variance is not possible within nationality and qualification groups because the number of candidates in some of these groups was very small compared to others. Also, no correlation is found between the scores and scale measures such as age and experience.
4. Discussion
Our findings suggest that there were some deficiencies in the storage of medicines in community pharmacies in Riyadh and probably in the delivery vehicles, especially during hot seasons. This may, at least partly, explain the existence of substandard levels of amoxicillin in Riyadh pharmacies [7]. Even basic thermometers were not available in about 13% of pharmacies, and in about 9% of the pharmacies equipped with a thermometer, the reading exceeded the 25˚C threshold. About 19% of pharmacies lacked a spare air-conditioner, making the quality of medicines questionable if the only available air-conditioner fails to operate optimally during summer. What is worse, some pharmacists reported that the air-conditioners may not have been kept running after working hours in hot seasons.
In addition, about 7% of refrigerators lacked thermometers, and in about 33% of refrigerators, the temperatures were outside the accepted range. In about 25% of the pharmacies, some walls behind medicine shelves were struck by direct sunlight from the outer side, and in about 4% of those pharmacies the walls felt warm.
The local regulations must be updated accordingly to ensure the best storage and distribution conditions for the medicines. Such detailed conditions and specifications may be obtained from the WHO [16,17]. The existence of an alternative power supply that covers the air-conditioning and refrigerators might be necessary in Riyadh although electricity blackouts are infrequent. However, if the durations of blackouts are enough to raise the temperature inside the pharmacy or the refrigerator above the allowed limits, then at least the refrigerator and one airconditioning unit must be linked to an alternative power supply in each pharmacy.
A significant percentage of pharmacists were unaware of the basic regulations relating to community pharmacy practice. The striking issue is that having knowledge about the regulations had no impact on the quality of storage in the pharmacies. This may explain the absence of any correlation between age or experience and the general score achieved. This also explains the lack of any significant differences between various grouping factors relative to the mean scores.
Stricter, periodic monitoring and inspection by the authorities are highly recommended. We suggest that all pharmacy owners must add spare air-conditioners as a prerequisite for licensing the pharmacies. Each pharmacy must be equipped with a room thermometer and a refrigerator thermometer that keep a record of temperature variation during the day. In addition, the pharmacy location must be in a position where its walls are not struck by direct sunlight from the outer side, or at least such walls must be adequately insulated. Medicine shelves must not be placed near the entrance or any location exposed to direct sunlight. Distributors’ storage and delivery facilities must also be strictly monitored.
Finally, the continuing education program for community pharmacists must be closely monitored and supervised by the authorities. We also recommend that passing an annual test about good pharmacy practice (GPP) should be a prerequisite for renewing the pharmacist license.
5. Conclusions
The quality of on-shelf medicines sold in community pharmacies in Riyadh may be questionable during hot seasons. Meanwhile, refrigerated medicines may not meet the quality standards throughout the entire storage time. More assertive measures and stricter monitoring of the adherence of the community pharmacies to good practice and good storage regulations are highly recommended. Community pharmacists’ continuing education and knowledge about the practice regulations must be improved.
6. Acknowledgements
The authors would like to thank the SFDA and the Saudi MOH for providing the necessary support and information about the pharmacies.
7. Authors’ Contribution
Research protocol development and manuscript revision: all authors. Field work: HMJK and HSA.
NOTES