1. Introduction
Developments in technology have led to the release of heavy metals such as lead, copper, chromium, nickel and zinc, which are hazardous to the environment and their toxicity and presence in the ecosystem poses a possible human health risk [1].
Lead is one of these heavy metals, and can be introduced to liquid wastes from the manufacturing processes of storage batteries, smelting and refining of lead and from the processes of mining. In water, lead tends to accumulate in aquatic organisms through the food chain and by direct uptake [2]. Lead is believed to cause hypertension, reproductive disorders and neurological and metabolic problems in humans [2].
Among the many methods available to reduce heavy metals concentrations from wastewater, the most common are chemical precipitation, ion-exchange, and reverse osmosis. Precipitation methods are particularly reliable but require large settling tanks for the precipitation of alkalines sludges and a subsequent treatment is needed [3,4]. Ion-exchange has the advantage of allowing the recovery of metallic ions, but it is expensive and sophisticated.
The adsorption process [5] is one of the most effective methods used to remove heavy metals from aqueous solution. Activated carbon is the most widely used adsorbent for this purpose because of its extended surface area, microporous structure, high adsorption capacity and high degree of surface reactivity. The commercial activated carbons are very expensive [6], this led to search for cheaper adsorbent. Consequently, numerous low-cost alternative adsorbent have been proposed including lingocellulosic wastes [7-11].
The agricultural wastes were considered as low-cost since they 1) require little processing and 2) are abundant in nature. Commonly, it concerns vegetal materials, then the term of biosorption is used to designate the fixation of contaminants onto biomaterials.
The main focus of this study was to evaluate the biosorption aptitude of a novel, low cost, and renewable biomass, Anethum graveolens for the removal of Pb(II) from aqueous solutions. The effects of pH, contact time, initial metal concentration and biomass dosage on the biosorption capacity were investigated. Moreover, various kinetic models were used to examine the experimental data. Experimental equilibrium data were fitted to the Langmuir, Freundlich, Temkin and Redlich-Peterson isotherm equations to determine the best-fit isotherm equation.
2. Experimental
Materials
Bio-dsorbent, Anethum graveolens, a desert plant is widely spread in Libya and Kingdom of Saudi Arabia. There is no previous report used Anethum graveolens as adsorbent material for removal of heavy metals. The roots were separated from the stems and leaves, washed with distilled water several times to remove the surface adhered particles and water soluble particles and dried at 80˚C in an electric oven for 24 h and ground using a mixer, and sieved to pass through a 150 - 200 mm. The roots were chosen because they contain the highest percentage of the cellulose content.
Reagents
Lead acetate, EDTA, ethanol, sodium carbonate and acetic acid were of analytical reagent grade supplied by Merck Company, Germany.
3. Methods
Bioadsorption Studies
The adsorbate solutions of 100 - 800 mg/l were prepared by dissolving certain weights of lead acetate in certain volumes of distilled water. The pH (2 - 4.5) of the solutions was adjusted with acetic acid or sodium carbonate solution. Equal volumes (100 ml of each) of the previously prepared metal ion solutions were placed in the corresponding number of 125 ml Erlenmeyer flasks each of which containing 0.05 g of the adsorbent and the whole flasks were shaken at 30˚C in a thermostatic water-bath at 150 rpm for 2 h. At the end of agitation time, the metal ion solutions were separated by filtration. The blank experiments were simultaneously carried out without the adsorbent. The extent of metal ion adsorption onto adsorbent was calculated mathematically by measuring the metal ion concentration before and after the adsorption through direct titration against standard EDTA solution. The amount of lead adsorbed, qe (mg/g) and percent removal of Pb(II) on Anethum graveolens were calculated according to the following equations:
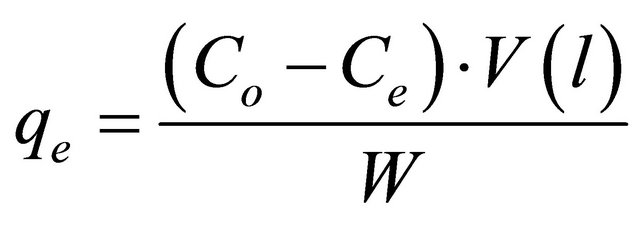 (1)
(1)
Percent Removal =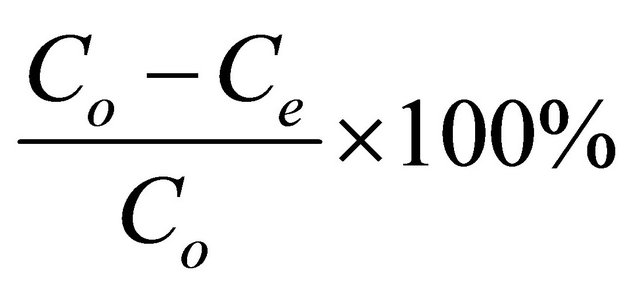 (2)
(2)
where Co and Ce are the initial and final concentrations of metal ion, mg/V is the volume of metal ion (l), W is the weight of bioadsorbent (g).
Analyses
Scanning electron microscopy (SEM)
To carry out an SEM analysis of Anethum graveolens biomass, the sample was first mounted on a standard microscope stub and coating with a thin layer of gold using a Polaron Diode Sputter unit. The analysis was performed using a JEOL JSM-15 scanning electron microscope.
Error analysis
In the single-component isotherm studies, the optimization procedure requires an error function to be defined to evaluate the fit of the isotherm to the experimental equilibrium data. The common error functions for determining the optimum isotherm parameters were, average relative error (ARE), sum of the squares of the errors (ERRSQ), hybrid fractional error function (HYBRID), Marquardt’s percent standard deviation (MPSD) and sum of absolute errors (EABS) [12]. In the present study, the average relative error (ARE) was used to determine the best fit in isotherm model as:
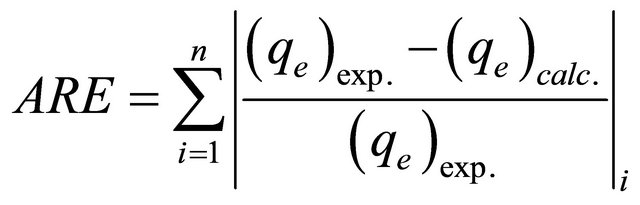 (3)
(3)
4. Results and Discussion
SEM of bioadsorbent
The SEM image (Figure 1) clearly shows that the sample of Anethum graveolens L is mainly composed of irregular and porous particles which indicated high surface area. It is clear from this figure that, Anethum graveolens has considerable numbers of pores where there is a good possibility of Pb(II) ions to be trapped and adsorbed into these pores.
Elemental analysis
The C, H, N contents of Anethum graveolens were analyzed with a Perkin-Elmer 240 CHN elemental analyzer. The element contents are as follows: C, 37.03%, H, 4.9%, N, 2.2%.
Factors affecting bio adsorption of Pb(II) onto Anethum graveolens
(Effect of initial pH)
The pH of the aqueous solution is an important controlling parameter in the adsorption process [13,14]. In the present work, adsorption of Pb(II) onto Anethum graveolens adsorbent was studied over the pH range of 2.0 - 4.5 for a constant adsorbent dose and constant concentration of adsorbate at 30˚C. As the acidity of the medium decreased, the extent of adsorption capacity, qe decreased (Figure 2). At high acidity, the Anethum graveolens particle surface will be completely covered with H3O+ ions and Pb(II) ions can hardly compete with

Figure 1. Scan Electron Microscope (SEM) of Anethum graveolens.

Figure 2. Effect of pH on adsorption capacity of Pb(II) onto Anethum graveolens at 30˚C.
them for adsorption sites. With the increase in pH, the competing effect of hydronium ions decreases and the positively charged Pb(II) ions adsorb on the free binding sites of the adsorbents. This is a common observation for all cases of adsorption of metal cations on solid surface in media of different acidity-basicity [15]. It is also significant that the active sites on the Anethum graveolens surface are weakly acidic in nature and with increase in pH, they are gradually deprotonated making available more and more sites for metal ion uptake [16]. At pH value higher than 4.5, the adsorption studied could not be carried out because metal ion will precipitate as lead hydroxide in this range.
Effect of adsorbent concentration (Adsorbent dose)
The effect of adsorbent concentration on both adsorption capacity and percent removal of lead are shown in Figure 3. It is clear from this figure that the percent removal of lead increases from 11% to 64% by increasing the concentration of adsorbent from 0.5 to 8 g/l and then remained at approximately the same level at higher adsorbent concentration. The increase in percent removal of Pb(II) with increasing adsorbent concentration in the first range could be attributed to the greater availability of the exchangeable sites of the adsorbent. The leveling of the percent removal at higher adsorbent concentration could be attributed to the blocking of the available active sites on the adsorbent surface. On the other hand, the adsorption capacity (qe), or the amount of Pb(II) adsorbed per unit mass of adsorbent (mg/g), decreases by increasing the concentration of adsorbent (Figure 3). The decrease in adsorption capacity with increasing the adsorbent concentration is mainly due to overlapping of the adsorption sites as a result of overcrowding of the adsorbent particles and is also due to the competition among Pb(II) ions for the surface sites [11].
Effect of contact time
Figure 4 shows the effect of contact time on the adsorption capacity, qe (mg/g) of Pb(II) onto Anethum graveolens at 30˚C using adsorbate concentration of 330 mg/l at fixed pH and at adsorbent concentration. Equilibrium adsorption was established after 15 min within the concentration range studied indicating that the adsorption rate is very fast. It is further observed that the adsorption curve is smooth and continuous, which indicate the possibility of the formation of monolayer coverage of Pb(II) ions onto Anethum graveolens This data is important because equilibrium time is one of the parameters for economical wastewater treatment.