Rendering of Cellulose Acetate Fabrics Self-Cleaning through Treatment with TiO2 Nano Particles ()
1. Introduction
Self-cleaning is a unique textile property which caught imagination of the consumer. Nanotechnology provides a new concept for production of self-cleaning textiles particularly by incorporation of TiO2 nanoparticles in the textile structure [1]. Nowadays, global commercial market of textile witness growing demand towards possessing extra functionalities for the fibres such as self-cleaning, antibacterial, environmental friendly and antipollution abilities [2].
It is anticipated that self-cleaning of fibrous materials such as cellulose acetate (CA) fabrics are important for their value added. To impart durable self-cleaning property to CA by applying TiO2 nanoparticles, it is a must to improve the adhesion between TiO2 and CA through alteration of the physical and chemical characteristics of CA surface. Laser irradiation pretreatment of CA surface is one technique to improve the bond-ability of TiO2 on CA fibers [3]. The surface pretreatment only modifies the outermost surface layers of the polymer without affecting the bulk properties. The UV absorption of titania-coated textiles is significant enough to promote excellent UV protection. No photo degradation of the molecular chains of textile fibres by the titania layers can be observed as demonstrated by a comparison study of the tearing strength of coated fabrics before and after light irradiation [4-6].
Sol-gel coating on textiles has been used to provide textiles with new properties, e.g. colouration, UV protection and medical applications [7]. A well-adherent surface of titanium oxide nanoparticles was produced on cellulose fibres at low temperature from an aqueous titania sol that was obtained via hydrolysis and condensation reactions of titanium isopropoxide in water [8]. An aqueous dispersion of nano-TiO2 was prepared and mixed with silicone softener to establish a finishing bath. Cotton fabrics were treated with this finishing bath as per a paddry-cure process. The so finished fabric was when exposed to UV irradiation; the UV/TiO2 process has been considered a promising technique for the decomposition of various contaminants [9].
Preparing nano-crystalline TiO2 and nano-crystalline anatase by sol-gel techniques to use them for coating the textiles was studied [1]. TiO2 colloidal solution was prepared by using an isopropanol-based sol-gel process, followed by hydrothermal treatment at 100˚C for 16 h and finally used to coat textiles. The long treatment time is not feasible in an industrial textile application and the use of organic solvents is not desired because it makes the whole chemical approach not environmentally friendly. More recently, self-cleaning cotton is prepared by coating it with single-phase anatase sols. These sols were synthesized by using a sol-gel process at low temperatures. The fibers coated with the anatase sol prepared at 60°C showed the highest photo catalytic activities. Anatase sol is also applied to functionalize textile fibers [10-12].
We undertake current work with a view to produce self-cleaning cellulose acetate fabrics through treatment of the latter with TiO2-nano-sol. Thus nanoparticles of TiO2 were dispersed in a mixture of water and ethylene glycol (1:1) and the treatment was performed as per the pad-dry-cure method. The treatment was carried out under a variety of conditions including concentration of TiO2-nanosol, curing time, microwave power, exposure time to UV radiation, incorporation of binder, pretreatment of CA fabrics with H2O2 prior to finishing treatment and comparison between fixation using microwave and thermofixation. Fabrics so treated were monitored for gain in whiteness index and loss in color strength as a measure of self-cleaning along with other properties, notably strength, roughness and wettability.
2. Experimental
2.1 Materials
White secondary cellulose acetate (CA), satin weave, of density 1.32 g/cm3 and of 38.5% acetyl content was used. The fabric was cleaned in an aqueous solution containing 2 g/l of nonionic detergent (Hostapal CV, Clariant) at 60˚C for 20 min followed by warm and cold rinsing. The fabric was dried under ambient conditions.
Titanium dioxide nano-powders of size <100 nm was supplied from Aldrich (Germany). All other used chemicals such as ethylene glycol, acrylate binder were of reagent grade.
Hydrogen peroxide (50%) was of laboratory grade chemicals. Egyptol® (nonionic wetting agent based on ethylene oxide condensate) was of technical grade chemicals. Commercial coffee used as received.
2.2. Methods
2.2.1. Pretreatment with H2O2
Cellulose acetate fabrics were treated in ultrasonic bath using a solution containing H2O2 (10g/l) along with an nonionic wetting agent (0.5 g/l) and sodium silicate (2 g/l). The treatment was carried out at 30˚C and pH 9 for 3 min. using a material to liquor ratio 1:20. The pH was adjusted using dilute orthophosphoric acid to avoid any possible degradation of treatment ingredients previously added. After every bleaching condition, the fabrics were subjected to thorough washing with water then dried at ambient conditions [13].
2.2.2. Treatment with TiO2 Nano-Sol
H2O2 treated CA fabric was dried at 100˚C for 5 min to remove the moisture content present in the fabric. CA samples were immersed in a mixture of water and ethylene glycol (1:1) containing the nanoparicles of TiO2 for 5 min. The nanoparicles concentration ranged from 0.5 to 1.5 g/100g fabric). Nanoparicles of TiO2 were dispersed in a mixture of water and ethylene glycol (1:1). The padded samples were then squeezed to a pick up of 100% and the dried at 70˚C for 10 min.
2.2.3. Microwave Treatment
Titanium dioxide treated CA samples along with the untreated sample were subjected to microwave irradiation as a means of curing using microwave oven [KOR-131 G(Olympic electric, Korea), 2450 MHz, 220 - 240 V, 50 Hz, microwave input power: 1350 W, microwave energy output: 1000 W, capacity 32 L]. The oven was operated at various power settings (80%, 90%, 100%) for different lengths of time (10, 20 and 30 sec). The cured fabric samples were rinsed (with distilled water) to remove the extra and unattached TiO2-nanoparticles followed by drying.
2.2.4. Staining Procedure
CA fabrics loaded with nano-TiO2 particles and those without these particles were stained by coffee and dried at 70˚C for 10 min. The fabrics were then exposed to UV irradiation using a UV light lamp (Philips TLO58W with a maximum intensity, wavelength (λ max at 365 nm). Irradiation was performed for different times (30, 60 and 90 minutes) at room temperature.
2.3. Measurements
2.3.1. Whiteness
Changes in whiteness index (W.I) of fabrics loaded with TiO2 nanoparticles and stained were measured before and after exposure to UV light, as a function for self-cleaning efficiency. Whiteness measurement was carried out using Ultra Scan PRO-Hunter Lab spectrophotometer according to AATCC test method 153 (1985) [14]. Gain of W.I was calculated as follows:

where W.I1 is the whiteness index before exposure to U.V irradiation;
W.I2 is the whiteness index after exposure to U.V irradiation.
2.3.2. Strength Properties
Fabric tensile strength test was conducted according to ASTM method 1682 (1994), which is a standard method for breaking force and elongation of tensile fabrics [15]. The width and the length of the fabric strip were 50 mm and 200 mm respectively.
2.3.3. Roughness Degree
Roughness degree of the treated (nano-TiO2 loaded) and untreated fabrics was measured using a surface roughness measuring instrument SE 1700 α (Japan). The device consists of two parts; one is connected to a digital screen and the second is connected to a movable sensor, the sample is fixed manually under the sensor; which moves on the sample surface for a distance of 4 mm and then records the mean result. This operation is repeated on different places for the same sample.
2.3.4. Wettability
Wettability, expressed as wetting time, of the treated samples was performed according to the standard method WT, AATCC Test Method 39 (1980).
2.3.5. Color Measurement
The color intensity, expressed as K/S value, of the stained samples before and after exposure to sun light was, as a function for self-cleaning efficiency, determined spectrophotometrically using Ultra Scan PRO-Hunter Lab spectrophotometer. K/S was calculated by applying the Kubelka-Munk equation [16]. Loss of K/S was calculated as follows.

where K/S1 is the color intensity before exposure to U.V irradiation;
K/S2 is the color intensity after exposure to U.V irradiation.
All the determinations in this work were done in triplicate and the results present mean values.
2.3.6. Scanning Electron Microscopy (SEM)
The surface morphology of untreated and treated fabric was investigated by using SEM, JSMT-20, JEOL-Japan. Before examination, the fabric surface was prepared on an appropriate disk and coated randomly by a spray of gold. SEM was carried out in National Research Centre (Egypt).
3. Results and Discussion
3.1. Tentative Mechanism
According to previous reports [8,17-19] preparation of TiO2-nanosol and the gel formation/fixation of titania cluster onto cotton fabric involve a number of interacttions that may be categorized and represented as follow:
1) Preparation of TiO2-nano sol [8]
 (1)
(1)
 (2)
(2)
and/or
 (3)
(3)
where R: is an organic group, and, Ti-O-Ti is a colloidal oxide network in the sol form.
2) Gel formation/fixation of titania-cluster onto cotton fabric [19].
 (4)
(4)
 (5)
(5)
 (6)
(6)
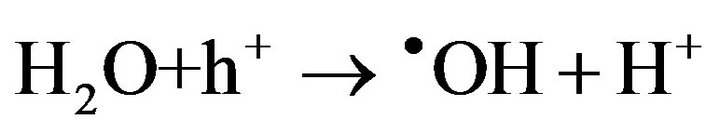 (7)
(7)
 (8)
(8)
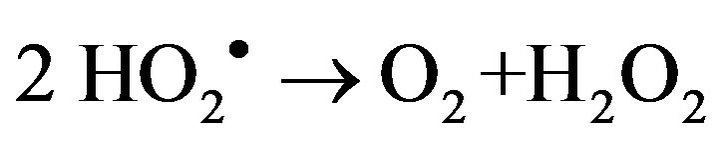 (9)
(9)
 (10)
(10)
 (11)
(11)
With the above mechanism in mind, parameters affecting the self-cleaning ability of CA fabrics loaded with nano-TiO2, expressed as gain of whiteness index and loss of color strength, are herein presented. Furthermore the onset of such Parameters include TiO2 nano sol concentration, curing time, microwave power, time of UV irradiation, incorporation of binder and microwave fixation. On the other hand, technical properties examined encompass strength properties, roughness and wettability.
3.2. Concentration of TiO2 Nano Sol
Table 1 shows the effect of TiO2 nano sol concentration on the self-cleaning ability and its onset on other technical properties of CA fabrics when the latter were treated with different doses of TiO2-nano sol using the pad-drycure method. Results of whiteness index gain and color strength loss make it evident that the self-cleaning ability of CA fabric is remarkably increased by increasing the TiO2-sol concentration from 0.5 g/100 g fabric to 0.75 g/ 100 g fabric. Further increase in TiO2 sol concentration causes marginal enhancement in self cleaning ability of the fabric within the range studied. At any event, however, the improvement in self-cleaning of CA fabrics is accompanied by substantial changes in their other technical properties. For instance warp tensile strength decreases slightly by increasing TiO2-nano sol concentration from 0.5% to. Thereafter the tensile strength decreases significantly and amounts to 27.5% at TiO2-nano sol concentration of 1.25 g/100g fabric. Elongation at break exhibits a value of 27% at TiO2-nanosol concentration of 0.5 g/100g fabric. Increasing the TiO2 concentration up to 1.25 g/100g fabric brings about slight decrease in elongation at break. Similar situation is encountered with roughness index. Wettability, on the other hand, decreases by increasing the TiO2-nano sol concentration within the range examined.
The foregoing concludes that CA fabrics loaded with TiO2 nanoparticles display self-cleaning properties, expressed as gain on whiteness index and loss in color strength (K/S). This is most probably owing to the high photo catalytic activity of the nano-sized TiO2 particles which enables generation of highly oxidative radicals onto titania film. In subsequent step, these radicals induce photodecomposition of the coffee stains on fabric surface loaded with nano-sized TiO2 particles and thus prevent them from built up [17,18].
Variations in tensile strength, elongation at break and roughness as well as wettability are a manifestation of the formation of nano-sized titania film on the fabric surface. This results in increments in both stiffness and covalent bonding between the uncondensed hydroxyl groups of titania and the hydroxyl groups of cellulose [11,14]. Indeed the observed decreased wettability by increasing TiO2-nano sol concentration is in conformation with thus.
3.3. Adjustment of Curing Time and Power Setting
Time and power settings of the microwave irradiation were investigated with a view of their adjustment. As shown in Table 2, gain in whiteness index percent increases by increasing the curing time from 10 to 15 seconds. Further prolongation of time has no perceptible increase in gain in whiteness index. The same holds good for the loss in color strength. The implication of this is that the time of curing should not exceed 15 second. Within a time ranging from 10 - 15 second highly acceptable self-cleaning properties could be achieved without strength, roughness and wettability deterioration.
Table 3 shows the effect of microwave power on selfcleaning properties of CA fabric loaded with nano-sized TiO2 particles and the impact of this on other technical properties of CA fabrics. Obviously, gain in whiteness index and loss in color strength, as a measure of selfcleaning ability of the nano TiO2-loaded fabric; display the most appropriate values at a microwave power of 90%. A microwave power of 100% produces similar results but with the certainty that other technical properties, notably, tensile strength decreases. Indeed, elevating the microwave power from 80% to 100% leaves the elongation at break, roughness and wettability practically intact. This indicates that the microwave power causes stabilization of CA fabric through gel formation/fixation of titania cluster and, in so doing, imparts self-cleaning properties without harming other technical properties. Nevertheless a microwave power of 90% is advocated for the next experiments as given below.