1. INTRODUCTION
Malignant breast cancer is the second leading cause of cancer death in women following lung cancer [1]. The modern era of breast cancer treatment has developed by great rapidity through the efforts of an extremely broad spectrum of clinical scientists whose efforts have redefined our standards for appropriate therapeutic strategies. Immunotherapy is a therapeutic strategy that manipulates immune responses against tumor cells; this type of therapy is a new area of cancer therapies which directed at passively or actively against tumor cells [2].
Dendritic Cells (DCs) are the most potent antigen presenting cells for naive T cell activation [3]. DCs originate from the bone marrow and reside in a resting or immature state in nonlymphoid tissues in which they efficiently capture and process antigens, Upon stimulation with bacterial products, inflammatory cytokines or CD40 ligation DCs undergo a maturation process that results in enhanced antigen presenting capacity and expression of MHC and costimulatory molecules and migration into secondary lymphoid organs in which they prime naive T cells [4-6]. The presence of DC at tumor site and regional lymph nodes pointed to a crucial role of these cells in antitumor immune responses [7,8]. Because of their unique capacity to stimulate resting T cells, DCs are the promising option for immunization protocols particularly for the induction of antitumor immunity to patients with malignant disease [9-11].
The rationale for this approach is based on the observation that DCs can be pulsed with tumor antigen and subsequently administered as a cellular vaccine to induce a specific antitumor response [12,13]. Methods that allow for the large scale in vitro generation of DC from peripheral blood monocytes using granulocyte-macrophage colony stimulating factor (GM-CSF) and interleakin-4 (IL-4) have developed and facilitated induction of immune responses in vitro as well as in clinical vaccination trials [14,15].
It is generally accepted that tumor growing in vivo naturally provide antigens to APC either by shedding from the surface of viable cells or by fragmentation of dead tumor cells. The previous studies have shown that multiple tumor antigens do exist which can be used to induce autologous tumor specific T cell responses in vitro, therefore an alternative strategy for effective vaccination may be the use of unfractionated tumor derived antigens such as tumor cell lysate [16], peptides eluted from tumor cell membrane [17], apoptotic tumor cell [18] tumor peptide pulsed DC derived exosomes [19] fusion of tumor and dendritic cells [20] and tumor cell RNA [21]. Among them whole tumor cells carry all the potential known and unknown tumor associated antigens (TAAs) that will be processed and presented on DC surface result in a polyclonal expansion of both CD4+ and CD8+ T cells [22], another advantages of using whole tumor cells as antigen is that unlike peptide antigens they can be used for patients with any HLA types. Furthermore, regardless of antigen types used in pulsing DCs, in the most cases this method of tumor antigen presentation could elicit tumor specific T cell responses in vitro [16- 21].
In the present study, we have evaluated whether tumor lysate pulsed DC derived from patients with breast cancer are able to elicit T cell responses in terms of proliferation, cytotoxicity and cytokine release against autologous tumor cells. Our aim was to obtain initial preclinical evidence for the potential efficacy of DC therapy.
2. MATERIALS AND METHODS
2.1. Media and Reagents
Complete medium (CM) was RPMI-1640 (Gibco, Germany) supplemented with 10% human AB serum (Blood Transfusion Organization, Tehran, Iran), 2.5 × 10–5 M 2 ME, 2 mM L-glutamine (Sigma Chemical Co, Munich, Germany), 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma Chemical Co, Munich, Germany). Organoid medium (OM) including DMEM supplemented with 2.5 × 10–5 M 2ME, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES, 0.075% bovine serum albumin (BSA), 0.5 µg/ml hydrocortisone, 5 µg/ml insulin and 5 µg/ml epidermal growth factor (EGF) (all from Sigma Chemical CO.-Munich-Germany) was used to culture freshly isolated breast normal and
tumor cells. Enzyme solution containing collagenase III (0.1 mg/ml), DNase (1 mg/ml) and hyaluronidase (1 mg/ml) (Sigma Chemical Co, Munich, Germany) was used for digestion of tumor and normal breast tissues. Recombinant human GM-CSF (Novartis-Basel, Switzerland), IL-4 and tumor necrosis factor alpha (TNF-a) (Peprotech, London-UK) were used to derive DC from peripheral blood monocytes and IL-2 (Peprotech-London, UK) to induce cytotoxic T lymphocytes (CTLs) from nylon wool enriched T cells. T cell proliferation assay (TPA) was performed by [3H] thymidine (Amersham Pharma, London, UK) uptake test. PKH-26 and Annexin V/PI fluorescent dyes (Sigma Chemical Co-MunichGermany) were used to label target cells and measurement of cytotoxic activity of T cells respectively. Finally, interferon gamma (IFN-γ) and IL-4 cytokines release assay was performed using commercially available ELISA kits (R & D Co. Stockholm-Sweden).
2.2. Patients
Tumor and normal tissue as well as peripheral blood were obtained from five patients who had undergone radical mastectomy for invasive ductal carcinoma of breast. Blood specimens were obtained at the time of surgery and two weeks later with weekly intervals (Surgery Department, Day Hospital, Tehran, Iran). Patients were 33 - 58 years old (mean = 43 ± 8 year) with stage III of disease (T3 N2 M0) and had received no treatment before surgery.
2.3. Preparation of Cell Suspension
Breast tumor and normal cells were isolated from fresh surgical specimens, single cell suspension were obtained by processing solid tissues under sterile condition at room temperature as described [23]. Briefly, tumor and normal tissues were mechanically minced to portions no larger than 1 - 3 mm3 in organoid medium (OM) and washed twice. The minced tissue were then placed into T25 culture flasks containing 5 ml enzyme solution and incubated overnight at 37˚C and 5% CO2. Enzymatically dissociated tissues were then applied to serial centrifugation to obtain cell suspension.
The resultant cells were washed twice in OM, counted and freezed in liquid nitrogen until use. When using, the cells were thawed and viable cells were enriched by percoll gradient centrifugation just before use. The natural killer (NK) sensitive target K562, a human eythroleakemia cell line (National Cell Bank, Tehran, Iran) was cultured in CM and used as target cell along with breast tumor and normal cells in T cell cytotoxicity assay.
2.4. Antibodies and Immunophenotyping
Flow cytometric analysis of monocyte derived DCs and primed T cells was performed by direct immunofluorescence staining of cell surface antigens using FITC or RPE conjugated mouse antibodies against CD1a, CD11c, CD14, CD83, HLA-DR, CD3, CD4, CD8 and appropriate isotype matched controls (Serotech London UK) Samples were analyzed on FACScan Calibur (Becton Dickinson USA) and CellQuest software.
2.5. Preparation of Tumor Cell Lysate
One to 2 × 107 tumor cells were washed in CM and subjected to four freeze (liquid nitrogen) and thaw (37˚C water bath) cycles to obtain a crude lysate. After removal of large particles by centrifugation (2000 g, 10 min followed by 13,000 g, 60 min, 4˚C) and sterilization by filtering (0.22 µm) the protein content was determined by the Cummassie blue protein assay (Biorad London UK) and aliquots stored at –80˚C until use.
2.6. Generation of Tumor Cell Lysate Pulsed DC
Monocyte derived DCs were generated as described [24]. Briefly, PBMCs were isolated on lymphoprep (1.077 g/ml Nycomed Pharma Oslo Norway) resuspended in CM supplemented with 10% AB serum and cultured in T25 flask for 2 hours at 37˚C, The nonadherent cells were then removed and used for T cell enrichment by nylon wool, the adherent cells were cultured in CM containing GM-CSF and IL-4 at final concentrations of 1000 U/ml and 800 U/ml respectively. Cultures were fed by removing 2 ml of medium and adding buck 3 ml fresh medium with cytokines every other day. On day 4 tumor cell lysate was added to immature DC to final protein concentration of 50 µg/ml and incubated overnight. Maturation factors including TNF-α with or without monocyte conditioned medium (MCM) (supernatant of overnight culture of autologous adherent PBMCs) 25% V/V were added on day 5. Tumor antigen pulsed mature DCs were harvested on days 7.
2.7. Autologous T Cell Proliferation Assay
T cell proliferation assay was performed using tumor lysate pulsed autologous DCs which were-irradiated to 3000 rad as stimulator and nylon wool enriched autologous T cells as responder cells in the ratios of 1:5, 1:10 and 1:20. A phytohemagglutinin stimulated T cells (2.5%) (Sigma Chemical Co Munich Germany) and DC and T cells alone were served as positive and negative controls respectively. Cultures were made in V bottom 96 well plates at final volume of 200 µl of CM supplemented with 10% AB serum for 5 days and [3H] thymidine was added at concentration of 1 µCi/well at last 18 hours of culture. Proliferative responses were measured by a liquid scintillation counter (Wallac Inc Turku Finland) and expressed as mean count per minute and stimulation index (SI) obtained for triplicate wells.
2.8. Tumor Specific CTL Induction
1 × 105 tumor cell lysate pulsed gamma-irradiated (3000 rad) DCs were cultured with 2.5 × 106 nylon wool enriched autologous T cells (ratio = 1:10) in CM supplemented with 10% AB serum in 24 well microtiter plate for 19 days at 37˚C in 5% CO2. Responder T cells were stimulated twice with tumor cell lysate loaded DC at a 1:20 ratio every other week. The culture were fed every 3 days with fresh medium and recombinant human IL-2 (20 IU/ml), the later was added after second round of restimulation. On day 19 tumor antigen primed T cells were harvested and subjected to phenotypic analysis using anti CD4 and anti CD8 antibodies.
2.9. T Cell Cytotoxicity Assay
Isolated breast tumor and normal cells were thawed and enriched for viable cells using percoll gradient centrifugation up to 80% - 90%. Fluorometric assessment of T lymphocyte antigen specific lysis was performed using PKH-26. Annexin V and propidium iodide (PI) fluorescence dyes with some modifications in the procedure described by Fisher [25]. Briefly, target cells including tumor and normal breast cells as well as K562 cell line were washed twice with serum-free medium before staining with PKH-26, the cells were then resuspended in loading buffer (Diluent C) and incubated for 60 minutes with 1 mM freshly prepared PKH-26 dye at 37˚C with shaking every 15 minutes. The staining reaction was stopped by incubation with 500 µl human AB serum for 30 s at room temperature. The cell pellet was transferred into a fresh 50 ml tube and washed twice with 40 ml CM containing 10% human AB serum. PKH-26 labeled target cells were incubated with primed T cells in 96 well V-bottom plates at effector: target ratio of 20:1 in a final volume of 200 µl at 37˚C for 3 hours. After co-incubation, cells were harvested and washed with phosphate buffered saline (PBS) and resuspended in 100 µl high calcium content Annexin V binding buffer. Staining with ann-FITC was performed for 20 minutes at room temperature in the dark immediately before flow cytometric analysis 1 µg/ml PI was added.
Three color FACS analysis was carried out on a Coulter cytometer (Coulter Co London UK). Unstained and PKH-26 stained as well as PKH-26 and Annexin V/PI stained target cells were used as controls. Duplicate wells were averaged and the percentage of specific lysis was calculated as follow:
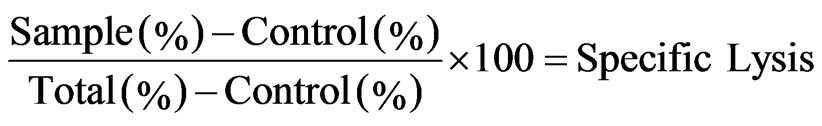
In this formula control and total indicates target cell lysis in the absence of effectors and target cells stained with PKH-26 alone respectively.
2.10. Cytokine Release Assay
Twenty four hours after last stimulation of autologous T cells with Tumor cell lysate pulsed DCs (described above), the supernatants were collected to measure IFN- γ and IL-4 release using commercially available sandwich ELISA kits as instructed in catalogue (R & D Co Stockholm Sweden). Cytokine release was reported as mean picograms ± SEM of IFN-γ and IL-4 for duplicate wells.
2.11. Statistical Analysis
The data depicted in each figure corresponds to one representative experiment of at least five independently performed experiments. Student’s t test was used to determine the significance of data comparison.
3. RESULTS
3.1. DC Generation
Human DC was generated from peripheral blood monocytes of 5 patients with stage III breast cancer (Table 1). Three days after culturing plastic adherent monocytes in the presence of GM-CSF and IL-4, clusters of nonadherent cells appeared and increased in size and number thereafter. Maturation factors (TNF-α, TNF-α and MCM) were added to tumor cell lysate pulsed immature DCs on day 5 and 60% - 70% of cells appeared as loosely adherent clumps or isolated floating cells with typical dendritic morphology on day 7, these cells exhibited typical cytologic features of DC i.e. large irregular cells with numerous cell membrane processes as reviewed by inverted light microscopy (Figure 1). The yield ranged 5% - 6.6% (mean = 5.8% ± 0.6%) of the plated PBMCs and the viability of harvested DCs was more than 98% as determined by trypan blue staining.
Flow cytometric analysis of DCs revealed significant differences in the expression of surface molecules crucially involved in DC functions. Compared to immature DCs, mature DCs consistently showed a substantially enhanced expression of HLA-DR and CD83 and decreased expression of CD1a, CD14 and CD11c, furthermore, TNF-α and MCM induced higher levels of expression of maturation markers such as HLA-DR and CD83 compared to TNF-α alone (Figure 2).
3.2. Autologous T Cell Proliferation
To address whether breast tumor antigen would able to induce proliferative response in T cells, nylon wool enriched autologous T cells were stimulated with tumor cell lysate pulsed DCs in 1:5, 1:10 and 1:20 ratios of stimulator to responder cells. Our results showed that T cell proliferative responses were elicited in three respective ratios for all 5 examined patients. However, proliferation rate and stimulation index varied from patient to patient. The lowest and highest stimulation index was 4.9 and 30 respectively (mean = 9 ± 3) (Figure 3).
3.3. T Cell Cytotoxic Activily
To examine the capacity of tumor cell lysate pulsed DC to elicit cytotoxic T cell response in vitro, we stimulated autologous T cells with antigen pulsed DCs three times by weekly intervals, and phenotypic and functional analysis was performed subsequently.
To determine the predominance of T CD4 or T CD8 proliferation during priming process, we compared the ratio of T CD4: T CD8 before and after priming of T cells. Our results demonstrated that this ratio increased in 3 (1.69 ± 0.21 to 3.1 ±1.5) and decreased in 2 (2.1 ± 0.2 to 1.67 ± 0.07) out of 5 patients (average: 1.85 to 2.55) (Figure 4).
Cytotoxicity assays were conducted with primed T cells at a minimum of 19 days after stimulation of T cell cultures with tumor lysate pulsed DCs. As shown in Figure 5 cytotoxicity against autologous tumor cells was demonstrated for all 5 patients at an effector: target ratio of 20:1 ranging from 13% - 76% (Med ± SEM = 41 ± 10%). Lysis of NK sensitive K562 cells was also observed (6.5% ± 21%), however lysis of autologous tumor cells was significantly higher than lysis of K562 cells

Table 1. Characteristics of patients with breast cancer.

Figure 1. Monocyte-derived tumor lysate pulsed dendrite ells. Peripheral blood mononuclear cells (PBMCs) from five patients with breast cancer were incubated for 2 hrs at 37˚C and adherent population were cultured in the presence of GM-CSF and IL-4. Immature DCs were pulsed with tumor antigen on day 4 and maturation factors (TNF-α plus monocyte condition medium (MCM) were added on day 5. Mature DCs were harvested on day 7 and reviewed by light microscopy.

Figure 2. Flow cytometric analysis of monocyte-derived DCs, Peripheral blood mononuclear cells (PBMCs) from patients with breast cancer were incubated for 2 hrs at 37˚C and adherent population were cultured in the presence of GM-CSF and IL-4, immature DCs were pulsed with tumor cell lysate on day 4 and maturation factors either TNF-α alone or TNF-α plus monocyte condition medium (MCM) were added on day 5. Tumor antigen pulsed mature DCs were harvested on day 7 and applied to FACS analysis using anti CD1a, CD11c, CD14, CD- 83 and HLA-DR antibodies (Percent of expressed cells (a), Mean fluorescent intensity (MFI) (b). *indicates the statistical significance (P ≤ 0.05).
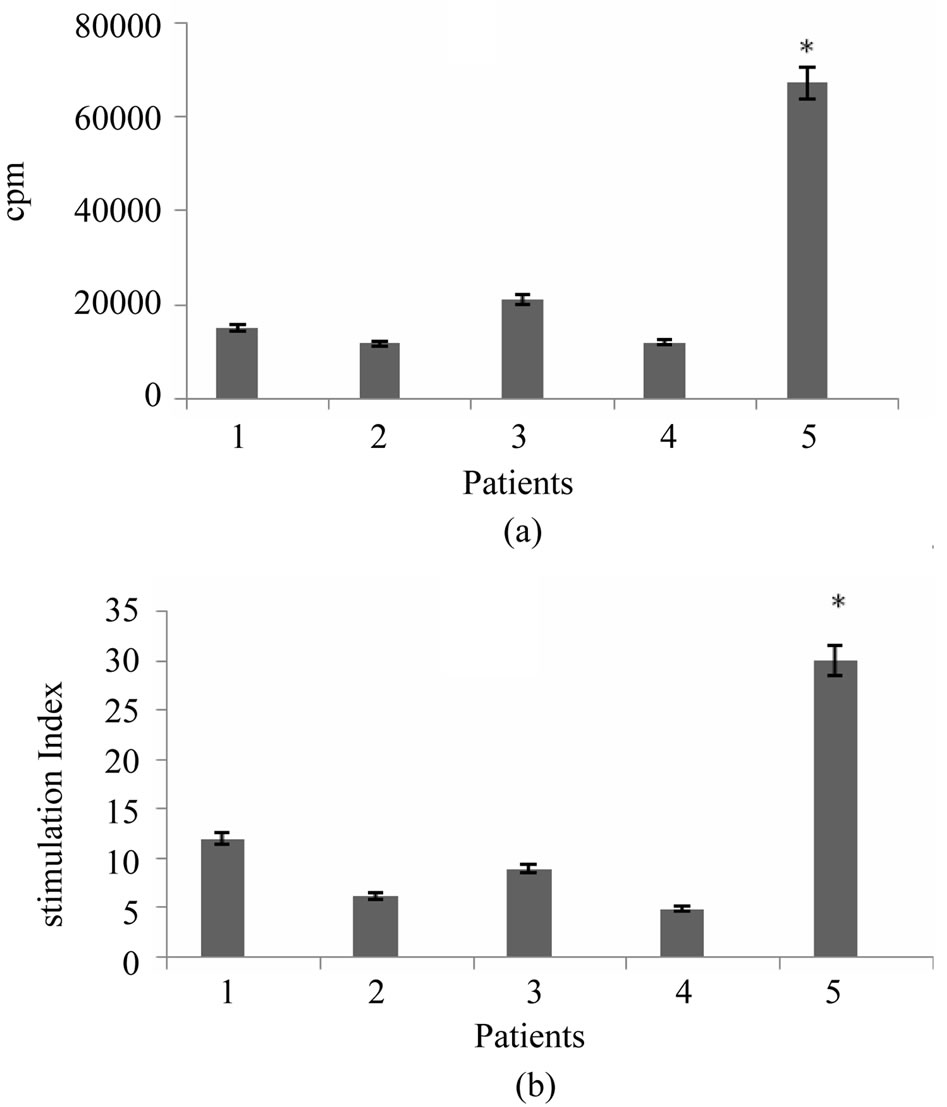
Figure 3. T cell proliferation response induced by tumor antigen pulsed DCs. Nylon wool enriched T cells were incubated with autologous breast tumor cell lysate pulsed DCs at a ratios of 5:1, 10:1 and 20:1 for 5 days. Uptake of [3H] thymidine during last 18 hrs of incubation was then measured. The results of T cell proliferation response are expressed as mean of triplicates. *indicates the statistical significance (P ≤ 0.05).

Figure 4. The ratio of CD4:CD8 in tumor antigen primed T cells. Autologous T cells from patients with breast cancer stimulated with breast tumor cell lysate pulsed DCs three times with weekly intervals, tumor specific primed T cells were subjected to phenotypic analysis using anti CD4 and CD8 monoclonal antibodies before (BP) and after priming (AP).
(P < 0.05). In contrast to the cytotoxic responses generated against autologous tumor cells, tumor antigen primed T cells failed to kill autologous normal cells of breast significantly (P < 0.05), the specific lysis against normal cells ranged from 0.6% - 8.3% (Med ± SEM = 0.8% ± 1.9%). Our findings also showed that the patients who expressed lower levels of TCD4: TCD8 ratio after
priming exhibited higher levels of cytotoxic activity (patients 2 and 3) and vice verse (patient 1, 4 and 5).
Collectively our results demonstrated that specific cytotoxicity against autologous tumor cells is a major component of tumor antigen primed T cells. Although an NK like cytotoxic response was also detected, autoreactive cytotoxic response against normal cells was mostly negligible (Figure 5).
3.4. Cytokine Release
It has been reported that tumor antigen primed T cells could secrete cytokines upon specific tumor lysate pulsed DC stimulation in vitro which appeared to correlate with their antitumor response efficacy. To address this issue, we examined cytokine profile produced 24 hours after third round of stimulation of autologous T cells with tumor lysate pulsed DCs in the supernatant of primed cells using commercially available sandwich ELISA. As shown in Figure 6, all 5 patients released variable amounts of either IFN-γ or IL-4 in response to restimulation, two of these patients secreted high levels of IFN-γ and 3 others secreted high levels of IL-4 (average of 367 pg/ml and 1725 pg/ml respectively). Interestingly, IFN-γ production by primed T cells was well correlated to high level of cytotoxic activity (r = 0.95) (Figure 7). Altogether tumor cell lysate pulsed DC stimulated T cells could elicit predominantly proliferation of T CD8+, specific cytotoxicity against autologous tumor cells and Th1 cytokine bias in some but not all patients with breast cancer.
4. DISCUSSION
We now believe that DCs play a unique role in antitumor immunity [26], they are potent inducers of CD4+ and CD8+ T cell mediated responses against tumor cells, in addition, evidences suggest that tumor antigen loaded DCs may elicit enhanced antitumor immunity in vitro as
well as in vivo [27].
The number of DCs in peripheral blood is not high enough to use in experimental or clinical settings, instead they generate large numbers of DCs from either bone marrow derived CD34+ precursors or peripheral blood monocytes [6,14]. Given to unique properties and potent ability of in vitro generated DCs to stimulate naive T cells, it is not surprising that different DC based protocols have already been applied to in vitro analysis or clinical trials for a number of cancers [2]. In the present study peripheral blood monocyte derived dendritic cells were used to load tumor antigens and stimulate T cell mediated responses in vitro in patients with breast cancer.
Morphological, phenotypic and functional characterization of monocyte-derived DCs were performed and revealed dendritic morphology with numerous cell membrane processes, CD14lo and CD83hi and HLA-DRhi expression and high level of mixed leukocyte reaction (MLR) stimulation index (data not shown). DC yield was 5% - 6% of initial culture of PBMCs which was comparable to results obtained by others [28].
Tumor specific immune responses to a variety of epithelial tumors have recently been generated in vitro from the peripheral blood of cancer patients [29,30]. In an attempt to perform a preliminary investigation on DC based breast tumor vaccine we examined T cell mediated responses induced by breast tumor antigen pulsed DC in

Figure 7. Specific lysis oe tumor antigen primed T cells which produced IL-4 or IFN-gamma predominantly. Autologous T cells from five patients with breast cancer were stimulated with breast tumor lysate pulsed DCs three times with weekly intervals and subjected to cytotoxic analysis as well as cytokine measurement in supernatant of T cells 24 hrs after third stimulation. *indicates the statistical significance (P ≤ 0.05).
vitro. In the last few years evidence that many murine and human tumors may express immunogenic tumor antigens that can be used as targets for tumor specific T lymphocytes has led to the development of different vaccination strategies for antitumor therapy. Because tumor specific antigens is frequently not available, most immunization approaches including this in vitro study have used DCs charged in vitro with whole tumor lysate [16, 28,30,31]. It should be noted that we performed our experiments under autologous condition using an experimental in vitro study in which DCs, T cells and breast tumor and normal cells were derived from same individual. Importantly, the use of tumor lysate has the potential advantages of allowing DC to present multiple immunogenic T cell epitopes without need for knowledge of the individual HLA type and including a broader TCD4+ and TCD8+ responses to tumor associated antigens, however, there is a few limitations such as little cross presentation of soluble antigens via MHC class I pathway as well as susceptibility to degradation by proteolytic enzymes present in the lysates.
DCs are capable to induce lymphocyte proliferation response especially when they are pulsed with the appropriate antigen. our previous experiment had shown that monocyte-derived dendritic cells from patients with breast cancer were capable to elicit mixed leuleocyte reaction (MLR) in vitro (data not shown), in the present study we demonstrated that tumor lysate pulsed DC could generate T cell proliferative response in vitro, proliferation induction by antigen loaded DCs, revealed that tumor lysate preparation would contain immunogenic epitopes that processed and presented by DC properly. In accordance to previous studies we showed that proliferative responses varied from patient to patient which expressed as different stimulation indexes [32], furthermore, the responses were increased by increasing the ratio of DC: T cells. Collectively, our findings as well as the results of other studies suggests that breast tumor antigens in the forms of tumor cell lysate or tumor-dendritic cells hybrid could elicit autologous T cell proliferative responses in vitro [25].
Phenotypic analysis of primed T cells at the end of 19 days culture and weekly stimulation with tumor antigen pulsed DC revealed that such a process results in predominantly proliferation of either TCD4+ orTCD8+ cells. In consistent to other studies [31], comparison of TCD4: TCD8 ratio before and after priming showed that it was increased in some patients [3] and decreased in others [2].
Our next approach was to determine the cytotoxic activity of tumor antigen primed T cells, there are numerous procedures to assay such an activity which 51Cr-release assay is known as golden standard test among them, however, in this study we used a simple and nonradioactive flow cytometric analysis of cytotoxic activity (see materials and methods) which is believed to be as sensitive and reproductive as 51Cr-release assay [25]. In order to mimic what may happen in patients immunized with DC bused vaccines, in this study we preferred to use unfractionated primed T cells as effector cells in cytotoxic activity assay, the target cells which labeled with PKH- 26 were autologous breast tumor and normal cells as well as NK sensitive K562 cell line.
Flow cytometric analysis of specific lysis using Annexin V and PI showed that tumor cell lysate pulsed autologous DC could elicit specific cytotoxic T lymphocyte response against autologous tumor cells (specific lysis = 13% - 76%). In all 5 patients with breast cancer, they failed to kill autologous normal cells (specific lysis = 0.6% - 8.3%) and exhibited variable amounts of cytotoxic activity against NK sensitive K562 cell line (specific lysis = 6.5% - 21%). Previous studies reported that tumor antigen pulsed DC primed T cells exhibited variable amounts of cytotoxicity in murine model [28] as well as in different cancer patients for example 20% - 60% in pancreas [34], 40% in multiple myeloma [35], 33% in melanoma and 41% - 58% in breast [30,33] cancers. It should be noted that in these studies specific lysis was determined by 51Cr-release assay which gives somehow lower cytotoxic activity values than flow cytometry.
In the case of cytotoxic activity a point that cannot be overlooked is that tumor lysate contain self antigens as well as tumor specific antigens, Although the benefit of using whole tumor cell lysate as antigen source for loading DC have been demonstrated in many cases without development of autoreactivity toward self antigens [36, 37]. Our potential concern is the possible induction of autoimmune reactivity to self or normal tissue antigens present in the tumor lysate as a consequences of processing by potent antigen presenting DC, however, our and others in vitro studies which used normal breast tissue, con A activated T cells or EBV transformed B cells as normal targets have shown negligible cytolytic activity against normal controls [30].
T cell mediated control of tumor is thought to be promoted by type 1 and impaired by type 2 cytokine response [37]. Recent studies, however, have shown significant dysfunction of type 1 T cell response in tumor bearing hosts [38]. Suggesting that tumor progression may be associated with preferential type 2 T cell responses. In this study we evaluated IFN-γ and IL-4 cytokine production by primed T cells as representatives of type 1 and type 2 cytokine patterns respectively. Our result showed that 2 out of 5 patients produced higher levels of IFN-γ and 3 others produced higher levels of IL-4 predominantly. These two patterns of cytokine production have been previously reported using either ELISPOT or cytoplasmic flow cytometry (CFC) analysis [30,39,40]. Taken together as reported previously, we found that breast tumor antigen pulsed autologous DC could elicit two patterns of immune responses in vitro, which generally known as Th1 and Th2 type response [30,34,39]. These patterns characterized by high level production of IFN-γ, predominantly proliferation of TCD8+ and strong specific cytotoxic activity against autologous tumor cells in one group (2 patients) and high level production of IL-4, predominantly proliferation of TCD4+ and modest to weak cytotoxicity against tumor cells in other group (3 patients).
In conclusion, our findings along with other studies [41,42] demonstrated that tumor cell lysate pulsed autologous dendritic cells could elicit T cell mediated immune responses in patients with breast cancer in vitro and in vivo. This approach may have important implication for the treatment of residual or resistant disease with active or adoptive immunotherapy after standard surgical and cytotoxic treatments, this modality particularly recommended to patients who induce type 1 immune response, however, the future design and implementation for clinical trial will ultimately determine the validity of this approach.
5. ACKNOWLEDGEMENTS
This work was supported by National Center of Medical Science Research (NCMSR) grant no. 2842. We grateful to Mrs. Nikoo Goftar at Transfusion Organization of Iran (TOI), Miss Hayat at the University of Iran Medical Science (UIMS) and Mrs. Sharifzadeh and her colleagues at Gamma-Irradiation Center of Atomic Energy Organization of Iran (GIC-AEOI).
NOTES