Acidic Polyester Imides as Thermally Stable Binder Polymers for Negative-Tone Black Photoresist ()
1. Introduction
Organic light emitting diodes (OLEDs) have become one of the major information displays, especially for the small size mobile displays owing to their lightweight, excellent form factor, wide viewing angle, low power consumption and high contrast ratio [1]. The panel of small size OLED has separate subpixels for the emission of red (R), green (G) and blue (B) light on the pixel define layer (PDL) which is fabricated by the photolithographic process utilizing positive-tone photoresist based on photosensitive polyimide with yellowish brown color. The light colored PDL surface of the OLED panel reflects ambient light thus requiring a 1/4λ polarizing film and black matrix (BM) patterning on the panel except for PDL blocks.
The use of negative-tone black photoresist instead of positive-tone photosensitive polyimide photoresist can simplify the OLED panel fabrication process by omitting the 1/4λ polarizing film and BM patterning. A typical negative-tone black photoresist includes photoinitiator, multifunctional monomers, black pigment millbase and binder polymer, which can be developed with aqueous alkaline solution in the photolithographic process [2]. The role of binder polymer is crucial in the patterning of black PDL on the OLED panel. It is not only one of the major components in the black photoresist but also affects the sharpness of the PDL patterns with geometry of about 5 × 5 μm per one subpixel during the development stage of the photolithographic process. After the photolithographic patterning of the PDL, the fabrication process temperature of the OLED panel reaches close to 300˚C. Therefore, the high thermal stability of the binder polymer is also essential.
A typical binder polymer employed in the negative-tone black photoresist is shown in Figure 1(a). This binder polymer so-called cardo-binder has been used in the patterning of black matrix (BM) and color filters of liquid crystal display (LCD). It is a polyester type epoxy acrylate containing fluorene moiety. It has two carboxyl groups and two acrylate groups per repeating unit of the polymer. The carboxyl groups make it possible to be developed by basic aqueous solution in the development stage of photolithographic process while the acrylate groups take part in the UV light initiated photo-crosslinking reaction in the UV exposure step. The cardo-binder is rather expensive, as noticed by the starting material and multiple steps of synthetic process (Figure 1(a)). Photocurable polyimides with double bonds have been reported as 3D printing materials [3] [4], and a chemically amplified poly(amic ester) binder polymer was reported using NMP/MeOH as a developer [5]. However, organosolubility, alkaline developability, chemical compatibility and thermal stability are the indispensable requirements for a qualified binder polymer in the black photoresist for patterning PDL on the OLED panel. It is usually challenging to synthesize UV-curable polyimides since the imidization reactions require such harsh conditions that acrylate or methacrylate type double bonds cannot withstand. In our past work, we reported a series of photosensitive polyimide/polyamic acid type binder polymers. A typical polyimide binder polymer is shown in Figure 1(b). It has a rather low molecular weight (Mw. below 3000) and 4 acrylate groups at its terminals [6]. Its acidity was provided by phenolic-OH groups. This polyimide based binder polymer exhibited higher thermal stability than the commercial cardo-binder but it had slight residue after development [7] [8].
![]()
Figure 1. Synthetic schemes of (a) cardo binder (CBP); (b) polyimide binder polymer (SAMG) with acrylate terminals.
In this work we studied a series of new polyester imide type binder polymers, which have two carboxyl and two acrylate groups per repeating unit while containing imide unit in the main chain. From the viewpoint of synthetic cost, we also designed a so-called one-pot synthesis of polyester imides which do not have any precipitation, filtration, evaporation step until the final step of obtaining polyester imide polymer solution.
2. Experimental
2.1. Materials
All the reagents and solvents were purchased from commercial sources and used directly in the experiments without further purification. Abbreviations of the compounds are as following: 4,4'-(Hexafluoroisopropylidene)diphthalic Anhydride (6FDA); 5-(2,5-dioxo tetrahydrofuryl)-3-methyl-3-cyclohexene-1,2-dicarboxylic anhydride (MCDA); 4,4'-Biphthalic anhydride (BPDA); trans-4-(aminomethyl) cyclohexanecarboxylic acid (CYAA); glycidyl methacrylate (GMA); fluorene epoxy acrylate (FEA); pentaerythritol triacrylate (PETA); 3,5-Di-tert-4-butylhydroxytoluene (BHT); tetrabutylphosphonium bromide (TBPB); dimethyl acetamide (DMAc); propyleneglycol monomethylether acetate (PGMEA).
2.2. Syntheses of Polyester Imides
One-pot solution polymerization of polyester imide homopolymers (FCYF and MBF). 6FDA (5 mmol) and CYAA (10 mmol) were mixed in PGMEA (30 wt% solid), stirred for 1 h at RT and then 23 h at 130˚C to form the diacid intermediate with imide cores. After cooling, into the above solution was added GMA (10 mmol) and extra PGMEA, and stirred at 110˚C for 3 h to form the diepoxymethacrylate (diol). Sequentially without separation of any components, into the solution was added 6FDA (4 mmol) and extra PGMEA, followed by stirring at 110˚C for 3 h. The final product solution (FCYF, 30 wt%) was cooled down and used in the photolithographic tests without further purification (Figure 2). In case of MBF, MCDA was first reacted with β-alanine to get the diacid intermediate with imide cores and the rest of the reactions were the same as the synthesis of FCYF.
![]()
Figure 2. Synthetic schemes of FCYF, and MBF polyester imides.
One-pot solution polymerization of copolymer type polyester imide (FSKF). Ethanolamine was mixed with PGMEA, and to this solution was added 6FDA (30 wt%). The mixture suspension was stirred at RT for 1 h, and then the temperature was raised to 130˚C while stirring for another 23 h to give diol intermediate with imide cores (FSIO). After cooling, 50 wt% FEA solution, 6FDA, BHT, TBPB and extra PGMEA were added to the FSIO diol solution and the mixture was stirred at 110˚C for 3 h. The final product solution (FSKF, 30 wt%) was cooled down and used in the photolithographic tests without further purification (Figure 3).
2.3. Characterization of Monomers with Liquid Chromatography-Mass Spectrometry (LC-MS)
For a typical LC-MS test, the PGMEA solution of diimide-diacid or diimide-diol intermediates were diluted with the eluent and injected into the column. Signals in terms of molecular weight in specific ranges of retention time were recorded.
2.4. Characterization of Polymers with Fourier-Transform Infrared Spectroscopy (FT-IR)
For a typical FT-IR test, excess hexane was added to the polyester imide solution and the precipitate was collected, followed by washing with hexane 3 - 4 times. The solid was dried at RT under vacuum overnight and subjected to KBr pellet preparation.
2.5. Evaluation of Thermal Stability with Thermogravimetric Analysis (TGA)
For a TGA test of UV-cured polyester imide samples, photoinitiator (SPI-03, 3 wt%) was added in the polyester imide solution and well dissolved. Then the solution was spin-coated on glass substrates, pre-baked (110˚C, 100 s), UV exposed (80 mJ) and subjected to post-cure (250˚C, 30 min). After cooling, the film on glass substrates was scratched off as powder for TGA test.
Powder samples were isothermally balanced in the TGA chamber at 100˚C under nitrogen atmosphere, after which the temperature was raised at a rate of
![]()
Figure 3. Synthetic scheme of FSKF polyester imide.
10˚C per minute. The thermal stability of each sample was represented by 3 typical parameters: 1 wt% loss temperature (1 WLT), 5 WLT and 500˚C residue (re.) as weight percentage (Figure 7).
3. Results and Discussions
3.1. Syntheses and Characterization of Polyester Imides
We used two approaches to synthesize polyester imides which can be used as binder polymers in the negative-tone black photoresist to fabricate micron size PDL pattern on the OLED panel. In Figure 2 is shown one approach in which dianhydride and amino acid type compound are reacted first to give diacid with two imide linkages in the core. This intermediate was reacted with glycidyl methacrylate (GMA) to give a diol compound with two acrylates. This diol was reacted with dianhydride again to give final product polyester imide.
The other approach is shown in Figure 3. Herein dianhydride was reacted first with alcohol amine compound to give a diol compound with two imide linkages in the core. This diol intermediate was mixed with another diol compound with two acrylates (FEA) which was used in the synthesis of a commercial cardo-binder as shown in Figure 1. The two diol co-monomers were reacted with dianhydride to give the copolymer type polyester imide.
It is noted that as for the dianhydride monomers for the synthesis of polyester imides 6FDA and MCDA were selected. In our previous work [6], it was found that these two dianhydride monomers could afford polyimides which could be completely soluble in common organic solvents, for example, propylene glycol methyl ether acetate (PGMEA), the major solvent used to make negative-tone black photoresist.
The diimide-diacid and diimide-diol intermediates had been analyzed by LC-MS. The MBC diimide-diacid showed a very clean signal representing its monovalent conjugate anionic base (Figure 4(a)); after reaction with GMA, the product gave signals of MBG itself and its fragment (Figure 4(b)).
The polyester imide products had been analyzed by FT-IR (Figure 5). Both FCYF and FSKF showed strong C=O stretching peaks at 1716 cm−1. Moreover, since 6FDA was used in both binder polymers twice, the -CF3 signals (1000 - 1400 cm−1) showed almost the same transmittance. The C-H stretching of methyl groups on FCYF was exhibited at 2933 cm−1.
Four typical polyester imide binder polymers showed Mw (Figure 6) from 1200 to 4000. Due to the less polarity of imide cores, the polymer chain may have smaller hydrodynamic volume in the GPC column, resulting in relatively lower Mw values.
3.2. Thermal Stability of Polyester Imides
The thermal stability of polyester imides were tested by TGA as described in the experimental Section 2.5. The TGA data are shown in Figure 7 and the evaluation is as follows.
![]()
Figure 4. LC-MS diagrams of (a) MBC; (b) MBG.
![]()
Figure 5. FT-IR graphs of FCYF and FSKF.
![]()
Figure 6. GPC graphs of (a) MBF; (b) FCYF; (c) FSKF and (d) CBP.
![]()
Figure 7. The TGA thermographs of selected polyester imides and a commercial cardo-binder (CBP).
1) Three polyester imides (FCYF, MBF, FSKF) exhibited 1 wt% loss temperature (1 WLT) lower than that of commercial cardo-binder polymer (CBP).
2) Of the three polyester imides FCYF has higher 1 WLT (294˚C) than the other two (MBF, FSKF). The low 1 WLTs of MBF and FSKF may be explained by the use of aliphatic β-alanine and ethanolamine in the synthesis of diol monomer with diimide core. While in case of FCYF, an amino acid (CYAA) containing cyclohexane moiety was used in the synthesis of diol monomers as intermediate, thus contributing to the increase of its thermal stability.
3) It is also noted that the polyester imide homopolymers (FCYF, MBF) had higher 1 WLTs than copolymer (FSKF). This may be due to the difference in reactivity of two diol monomers (FSIO and FEA) which may cause accumulation of thermally weak repeating units in the copolymer chain.
4) The CBP cardo-binder is homopolymer and the fluorene unit is well-known to give high thermal stability, thus contributing to high 1 WLT value.
5) The good thermal stability of FCYF polyester imide is also noted by its high 5 WLT (356˚C) compared to the 353˚C of CBP.
3.3. Photolithographic Tests
The acidic polyester imide binder polymers were subjected to photolithographic tests as shown in Table 1. The black photoresists were spin-coated on a SiO2 (200 nm)/Si wafer, soft-baked, exposed with UV lamp under a photomask, post-baked and finally developed with TMAH (2.3 wt%) aqueous solution at room temperature.
In photolithographic tests, black photoresist containing MBF showed only a straight band (no micron size box pattern), presumably due to over-crosslinking during the UV exposure caused by its high double bond density (DBD, mmol of double bonds in 1g of polymer) of 1.76 mmol/g and less steric hindrance near double bonds. The black photoresist with commercial binder polymer CBP gave good PDL box pattern as shown in Figure 8(a). Since FCYF had longer and more flexible polymer chain, as well as bulky -CF3 groups, it could be more compatible with the black millbase, so acceptable patterns were formed (Figure 8(b)). In case of FSKF, it gave better PDL pattern than FCYF probably due to the presence of FEA moities which is known to help good dispersion of black millbase pigment (Figure 8(c)).
![]()
Table 1. Black photoresist formulations with synthesized polyester imides, and CBP as reference binder polymer.
aPM6:
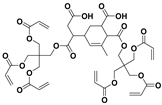 ; b3-(trimethoxysilyl) propyl methacrylate.
; b3-(trimethoxysilyl) propyl methacrylate.
![]()
Figure 8. The optical microscope images of photolithographic patterns using photoresists: (a) PR-0; (b) PR-1; and (c) PR-2.
4. Conclusion
In this work various synthetic methods were developed to obtain good binder polymers which have high thermal stability, high compatibility with the other components of the black photoresist, and fine photolithographic patterning property for the negative-tone black photoresist. The syntheses of diimide-diacid or diimide-diol intermediates for the polyesterification with dianhydride gave polyester imides which could meet the above requirement. It is also noted that these polyester imides were synthesized by one-pot reaction which does not involve any precipitation, filtration, separation or distillation in the synthetic process. We believe that this work will give rise to a good idea to the design and optimization of thermally-stable binder polymers for the negative-tone black photoresists.
Acknowledgements
This work was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program (10063289), Development of High Temperature Negative tone Photosensitive Black Resin and Fabrication Process for Pol-less AMOLED Devices) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).