Effect of Microwave Irradiation on Friedel-Crafts Diphenylmethylation of Arenes ()
1. Introduction
Recently, environmental problems such as global warming caused by energy consumption have garnered concern [1] [2] . As such, it is necessary to develop efficiently and environmentally friendly reactions in synthetic organic chemistry. Towards that end, improvements in reaction yield and selectivity under microwave irradiation compared to conventional heating have been reported [3] [4] .
Diarylmethane and triarylmethane derivatives are important skeletons in various functional materials and biologically relevant substances [5] . They are generally synthesized by the reaction between benzyl derivative 1 as an electrophile and arene 2 as a nucleophile (Scheme 1). With regard to the synthesis of compound 3, direct arylation via Friedel-Crafts using a Lewis acid catalyst has been
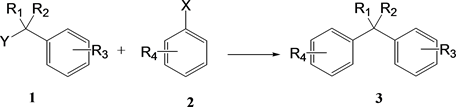
Scheme 1. The reaction between benzyl derivatives and arenes.
reported [6] . The Friedel-Crafts reaction is a typical carbon-carbon bond forming reaction used in synthetic organic chemistry. Mechanistically, the Lewis acid coordinates to the haloalkane to give an alkyl cation. Thereafter, the alkyl cation electrophilically attacks the arene to yield a product with a new carbon-carbon bond. Among Friedel-Crafts reactions, reactions with alcohols are environmentally friendly because the by-product is water.
In regard to viable catalysts for Friedel-Crafts reactions, various Lewis acids [7] , Bronsted acids [8] , and transition metal complexes [9] have been developed. However, such catalysts are often expensive, toxic, and air-sensitive. Therefore, reactions using iron as a Lewis acid catalyst have attracted attention, because iron is inexpensive and relatively environmental-friendly [10] [11] .
In this study, a Friedel-Crafts diphenylmethylation reaction using iron(III) chloride as a catalyst was developed for the environmentally friendly synthesis of triarylmethane derivatives. The reactions were carried out under conventional heating and microwave irradiation, and a mechanism was proposed.
2. Experimental
2.1. Instruments
GC data were acquired using a Shimadzu GC-14B with FID detector. Separation of the compounds was carried out using a Shimadzu HiCap-CBP10 (0.2 mm I.D. × 25 m, 0.25 μm film thickness) capillary column, and the carrier gas was nitrogen. The GC oven was programmed at 240˚C - 270˚C.
2.2. Substrate
The substrates were purchased from Wako Chemicals, Ltd. The synthesized triarylmethanes are shown in Scheme 2.
2.3. Friedel-Crafts Reaction
Diphenylmethanols (0.2 mmol) and substituted benzenes (0.2 mmol) were dissolved in 20 mL of cyclohexane in the presence of iron(III) chloride (0.2 mmol). The reaction flask was irradiated with 300 W microwaves. The reaction temperature increased to 35˚C. After the reaction, 30 mL of water was added to the flask, and the reaction mixture was extracted with 20 mL of ethyl acetate. The reaction yields were determined by GC.
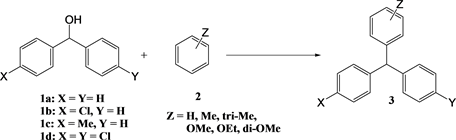
Scheme 2. The synthesized triarylmethanes.
For the reactions with diphenylmethanol and 4-chlorodiphenylmethanol, dichloromethane was used as the solvent. In this solution, the temperature reached the boiling point of the solvent.
The reaction temperatures under conventional heating conditions were 35˚C in cyclohexane and reflux in dichloromethane.
The formed triarylmethanes were identified by 1H and 13C NMR. The yield was determined using GC.
3. Results and Discussion
3.1. Reactions between Diphenylmethanols and Substituted Benzenes
Reactions between diphenylmethanol and alkylbenzenes were carried out in dichloromethane (Table 1). In dichloromethane, no differences in the yields were observed under conventional heating or microwave irradiation, which was likely due to the absorbance of the microwaves by dichloromethane; as such, the substrate could not efficiently absorb the microwaves.
Therefore, the reactions were carried out in cyclohexane, which has a lower polarity than dichloromethane. However, no reaction occurred with the alkylbenzenes, so the reaction was attempted with several alkoxybenzenes. The yields of the reactions between diphenylmethanol and alkoxybenzenes are shown in Table 2.
With anisole and phenetol, the product yields under conventional heating were 48% and 50%, respectively. Under the microwave conditions, the yields increased to 76% and 62%, respectively. These results indicated the existence of a microwave irradiation effect.
On the other hand, with 1,2-dimethoxybenzene and 1,4-dimethoxybenzene, there was no difference in the yield, perhaps due to the lower reactivity of these alkoxybenzenes.
The results of the reactions with 4-chlorodiphenylmethanol are summarized in Table 3. The yields with 4-chlorodiphenylmethanol decreased compared to those with diphenylmethanol, likely because of the inefficient coordination with iron(III) chloride. However, the accelerating effect of the microwaves was observed with anisole and phenetol, similar to the case with diphenylmethanol. However, when using 1,2-dimethoxybenzene and 1,4-dimethoxybenzene, no
![]()
Table 1. The yield of the reaction between diphenylmethanol 1a in dichloromethanea.
a. [1] = [2] = [FeCl3] = 10 mM, 0.2 mmol, b. Yield determined by GC.
![]()
Table 2. The yield of the reaction between diphenylmethanol 1a in cyclohexanea.
a. [1] = [2] = [FeCl3] = 10 mM, 0.2 mmol, b. Yield determined by GC.
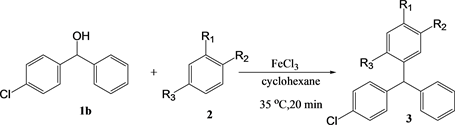
![]()
Table 3. The yield of the reaction between 4-chlorodiphenylmethanol 1b in cyclohexanea.
a. [1] = [2] = [FeCl3] = 10 mM, 0.2 mmol, b. Yield determined by GC.
difference in the yields was found under conventional heating or microwave conditions.
There was no difference in the yields in the reactions with 4-methlydiphe-nylmethanol under conventional heating or microwave conditions, owing to the high reactivity caused by the high electron donating effect of the methyl group (Table 4).
The microwave irradiation effect was observed in the reactions with 4,4’-dichlorodipenylmethanol, as well as for the reactions with diphenylmethanol and 4-chloromethanol (Table 5). The results using 1,2-dimethoxybenzene and 1,4-dimethoxybenzene were also similar to those for diphenylmethanol and 4-chloromethanol.
3.2. The Effect of Amount of Iron(III) Chloride on the Reaction between Diphenylmethanol and Anisole
The amounts of iron(III) chloride were varied in the reaction between diphenylmethanol and anisole (Figure 1). With 0.2 - 0.6 equivalents of iron(III) chloride, no difference was found under conventional heating or microwave irradiation. However, in the presence of 0.8 equivalents of iron(III) chloride, the
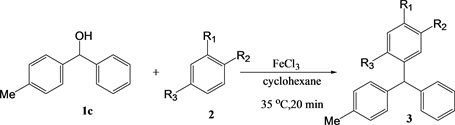
![]()
Table 4. The yield of the reaction between 4-methyldiphenylmethanol 1c in cyclohexanea.
a. [1] = [2] = [FeCl3] = 10 mM, 0.2 mmol, b. Yield determined by GC.
![]()
Figure 1. Amounts of iron chloride used in this reaction: comparison between Conventional heating and Irradiation of microwave.
accelerating effect of the microwave was observed. Based on these results, an equivalent amount of iron(III) chloride was necessary for the accelerating effect.
3.3. Temperature of Reaction System
The reaction temperature versus irradiation time plot is shown in Figure 2, where the amount of the substances was fifty times that in the reaction. For the
![]()
Figure 2. The temperature versus irradiation time.
substrates alone or iron(III) chloride alone, the reaction temperature was 24˚C; no temperature increase was observed (Figure 2(a)). However, when the substrates and iron(III) chloride were combined, the temperature increased to 40˚C (Figure 2(b)), suggesting that the microwave energy was absorbed by the complex formed between the arenes and iron(III) chloride (Figure 3).
4. Conclusion
In cyclohexane, which is a low polarity solvent, microwave irradiation was found to accelerate the reaction between diphenylmethanol and alkoxybenzenes. However, when methylene chloride, which has a higher polarity, was used, no differences in the product yields were observed under conventional heating or microwave irradiation, perhaps due to the fact that methylene chloride absorbs the microwaves, and the substrates were not affected by the microwave energy. Furthermore, when more than 0.8 equivalents of iron(III) chloride were used, an accelerating effect was observed under microwave irradiation. Moreover, when
![]()
Figure 3. The complex between the arene and iron(III) chloride.
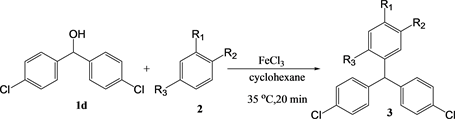
![]()
Table 5. The yield of the reaction between 4,4’-chlorodiphenylmethanol 1d in cyclohexanea.
a. [1] = [2] = [FeCl3] = 10 mM, 0.2 mmol, b. Yield determined by GC.
the arenes and iron(III) chloride were present, the reaction temperature increased to 40˚C. Therefore, iron(III) chloride plays an important role in accelerating the reaction. Namely, the chemical species formed upon coordination of iron(III) chloride to the arenes selectively absorbs the microwaves. Therefore, local heating around these chemical species would occur, and the acceleration effect would be affected by the increased temperature.
NOTES
1H and 13C NMR were measured in chloroform-d at room temperature using a JEOL A-400 spectrometer.