Some New Heterocycles from the Reaction of 4-Carboxaldehyde-2-Phenyl-2H-1,2,3-Triazole Malononitrile ()
1. Introduction
Our continuous studies have demonstrated the utilization of carbohydrates specially L-ascorbic acid and its analogs in the synthesis of heterocycles including pyrazoles, triazoles, imidazoles and isoxazolines [ 1 - 5 ]. The observation that triazole and pyrazole derivatives are biologically active compounds [ 6 - 10 ] specially as insecticides and antiflamatory has promoted the extension of our study to synthesize a new series of this class of compounds in the hope of producing more effective chemotherapeutic agents.
2. Results and discussion
Condensation of 4-carboxaldehyde-2-phenyl-2H-1,2,3-triazole 1 (obtained from the oxidation of D-glucosozone with cupper sulphate followed by periodate oxidation) with molononitrile in the presence of triethylamine, afforded the triazolomethylene malononitrite 2 whose 1H NMR spectrum showed two singlets at δ 7.99 and 8.53 for the ethylenic proton and the C5-H triazole ring respectively. Treatment of 2 with hydrazine hydrate in presence of piperidine, afforded the bicyclic compound, namely, 3-amino-5-(2-phenyl-2H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazole-4-carbonitrile 3 (Scheme 1) whose structure was confirmed from its spectra and elemental analysis. Its 1H NMR spectrum showed a singlet of two proton intensity at δ 5.35 due to NH2 group, a doublet at δ 7.68 (C5'-H) a doublet at 8.33 (C4'-H) and a ginglet at 8.41 (C5-H). The reaction of 2 with hydroxylamine hydrochloride, afforded the bicyclic compound 4 namely, 5-amino-3-(2-phenyl-2H-1,2,3-triazol-4-yl)-2,3-dihydroisoxazole-4-carbonitrile whose structure was confirmed by 1H NMR which revealed a singlet at δ 5.53 due to NH2 group , a singlet of one proton intensity at δ 7.56 (C5-H) and a singlet at δ 8.39 (C5-H). Furthermore treatment of 2 with thiourea in the presence of piperidine, gave 2,4-diamino-6-(2-phenyl-2H-1,2,3-triazol-4-yl)-6H-1,3-thiazine-5- carbonitrile 5 whose structure was also confirmed by elemental analysis and NMR data. The 1H NMR showed two singlets of one proton intensity each at δ 8.20 (C6-H) and 8.48 (C5-H). The reaction of 2 with ethanol amine, afforded 2-amino-4-(2-phenyl-2H-1,2,3-triazol-4-yl)nicotinonitrile 6 whose 1H NMR revealed two doublets of one proton intensity each at δ 7.25 (C5`-H) and 7.89 (C5-H). Moreover, treatment of 2 with thiosemicarbazide in presence of piperidine, gave 4-amino-2-hydrazinyl-6-(2-phenyl-2H-1,2,3- triazol-4-yl)-6H-1,3-thiazine carbonitrile 7 whose structure was confirmed by 1H NMR which showed three singlets of one proton intensity each at δ 8.20 (C6-H), 8.60 (C5-H) and 11.69(NH). Finally treatment
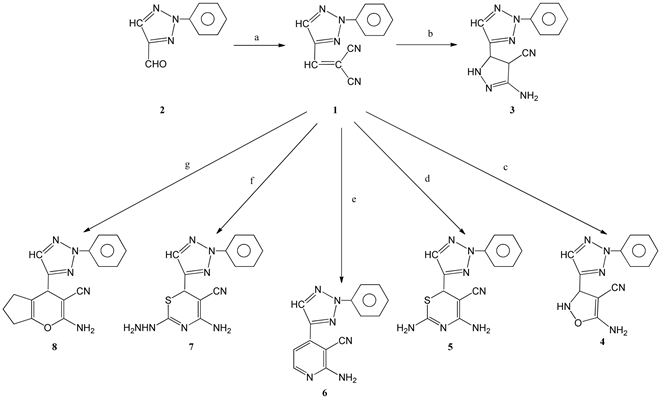
Scheme 1. Reagents and conditions: (a) CH2(CN)2, EtOH, triethylamine, Δ, 2 h; (b) NH2NH2・H2O, EtOH, piperidine, Δ, 2 h; (c) NH2OH・HCl, EtOH, piperidine, Δ, 2H; (d) NH2CSNH2, EtOH, piperidine, Δ, 2 h; (e) NH2CH2CH2OH, EtOH, piperidine, Δ, 2 h; (f) NH2NHCSNH2, piperidine, Δ, 2 h; (g) cyclopentanone, EtOH, piperidine, Δ, 2 h.
of 2 with cyclopentanone-gave 2-amino-4-(2-phenyl-2H-1,2,3-triazol-4-yl)-4,5,6,7-tetrahydrocyclo-penta [b] pyran-3-carbonitrile 8. Its structure was established from its elemental analysis and 1H NMR data which revealed two singlets of one proton intensity each at δ 8.42 (C5-H) and 8.57 (C5'-H) in addition to the expected proton signals. The reaction course leading to compounds 3-7 Scheme 2 from the triazole methylenemalononitrile can be assumed to proceed via the addition of the mercapto or amino groups at the ethylenic double bonds and thne nucleophilic attack of the amino or imino groups to the cyclized product except for the reaction of ethanol-amine, the reaction may proceed through the elimination of water molecule and nucleophilic attack of the amino group followed by elimination of H2 by auto oxidation. In addition, the reaction course leading to the formation of 8 from the triazole methylenemlonnitrile Scheme 2 can be assumed to proceed through the formation of cabanion fallowed by nucleophilic addition at the ethylenic bond and cyclization to give 8. The reaction of 4-carboxaldehyde-2-phenyl-2H-1,2,3-triazole 1 with hydrazine hydrate, afforded 4-carboxaldehyde-4-hydrazone 9 which upon treatment with equivalent amount of 1 afforded 1,2-bis[(2-phenyl-2H-1,2,3-triazol-4-yl) methylen] hydrazine 10. Similarly treatment of 9 with 3-carboxaldehyde-1-phenyl-4,5-pyrazolinedione-4-phenylhydrazone, gave 1-phenyl- 3-[2-phenyl-2H-1,2,3-triazol-4-yl)-methylenehydrazonomethyl]-4-(phenylhydrazono)-1H-pyrazol-5(4H)- one 11 (Scheme 3).
3. Experimental
3.1. Apparatus and Materials
Melting points were determined on a Kofler block and tottoli (Büchi) apparatus and are uncorrected.
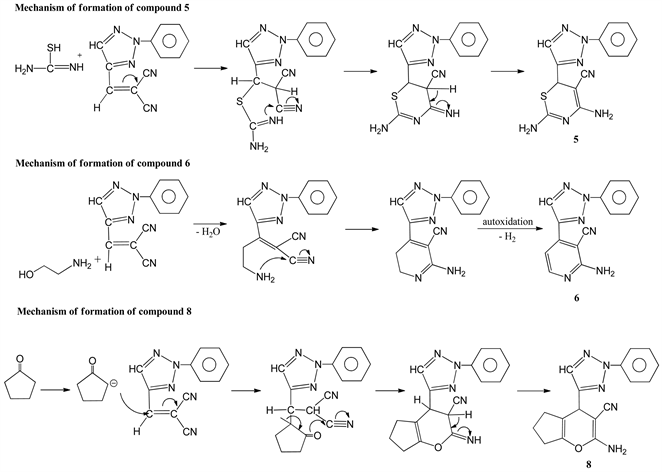
Scheme 2. Mechanisms of formation of compound 5, 6 and 8.
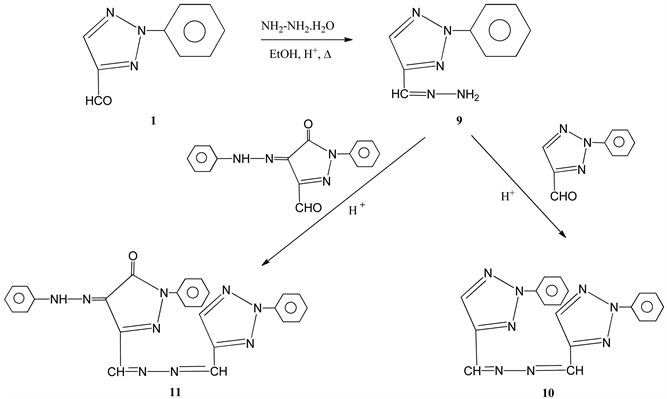
Scheme 3. Formation of compounds 9, 10 and 11.
Elemental analyses were carried out in the microanalytical laboratory of the Faculty of Science, Cairo University. The IR spectra of compounds were recorded on a Perkin-Elmer 580B spectrophotometer as potassium bromide pellets and frequencies are reported in cm−1. The 1H NMR spectra were recorded on a Cameca 250 NHz spectrometer using TMS as internal standard. Mass spectra were recorded with LKB model 2091 spectrometer and intensities are given in parentheses as percentage of the base peak.
2-(2-Phenyl-2H-1,2,3-triazol-4-yl) methylenemalonomitrile (2) A solution of 1 (2 g, 11.5 mmol) in absolute ethanol (30 ml) was treated with malononitrile (0.94 g, 12 mmol) and few drops of triethylamine. The reaction mixture was heated under reflux for 2 h, concentrated and left to fool. The yellow solid was filtered off, washed with ethanol and dried (1.85 g; 71%). It was recrystallized from ethanol as yellow needles m.p. 133˚C - 134˚C; νmax/cm−1 2233 (
) and 1609 (C=N); NMR: δH(CDCl3) 7.99 (s, 1H, ethylinic proton), 7.40 - 8.14 (m, 5H, aromatic-H), 8.53 (s, 1H, C5-H); 13C NMR (CDCl3): δ 147, 138, 137, 119, 112, 84. Anal. Calc. for C12H7N5: C, 65.16; H, 3.17; N, 31.67. Found: C, 65.32; H, 4.04; N, 31.87%.
3.2. General Procedure
A mixture of 2-(2-phenyl-2H-1,2,3-triazol-4-yl) methylenemalononitrile 2 (0.3 g; 1.35 mmole) in absolute ethanol (20 ml) was treated with an equimolar amount of hydrazine hydrate, hydroxylamine hydrochloride, thiourea, ethanol amine, thiosemicarbazide or cyclopentanone and few drops of piperidine. The clear solution was heated under reflux for 2 h, concentrated, poured onto ice water and neutralized with acetic acid. The solid obtained was filtered off, washed with cold water, ethanol and dried. Each product was recrystallized from ethanol in needles.
3-Amino-5-(2-phenyl-2H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazole-4-carbonitrile (3): (0.26 g; 81%); m.p. 180˚C - 181˚C; IR: νmax/cm−1 3213 (NH2), 2236 (
) and 1612 (C=N); NMR: δH(CDCl3) 5.35 (s, 2H, NH2), 6.19 (s, 1H, pyrazole H), 7.39 - 8.17 (m, 5H, aromatic-H), 7.68 (d, 1H, j5,4 = 9.4 Hz, C5'-H), 8.33 (d. 1H. J5,4 = 7.42 Hz, C4'-H), 8.41 (s, 1H, C5-H). 13C NMR (CDCl3): 153, 136 - 134 (aromatic C), 133, 129, 128, 119. Anal. Calc. for C12H11N7: C, 56.92; H, 4.35; N, 38.73. Found: C, 56.78; H, 4.42; N, 38.52%.
5-Amino-3-(2-phenyl-2H-1,2,3-triazol-4-yl)-2,3-dihydroisoxazole-4-carbonitrile (4) (0.3 g; 76%): m.p. 185˚C - 186˚C; IR: νmax/cm−1 3417 (NH), 2221 (
) and 1625 (C=N); NMR: δH(CDCl3) 5.35 (s, 2H, NH2), 7.42 - 8.51 (m, 5H, aromatic-H), 7.65 (s, 2H, NH2), 7.65 (s, 1H, C5'-H), 8.39 (s, 1H, C5-H), 9.26 (s, 1H, isoxazole-NH). Anal. Calc. for C12H10N6O: C, 56.69; H, 3.94; N, 33.07. Found: C, 56.46; H, 3.78; N, 33.21%.
2,4-Diamino-6-(2-phenyl-2H-1,2,3-triazol-4-yl)-6H-1,3-thiazine-5-carbonitrile (5) (0.3 g; 72%); m.p. 193˚C - 194˚C; IR: νmax/cm−1 3362 (NH), 2236 (
), and 1638 (C=N); NMR: δH(DMSO-d6) 7.47 - 8.05 (m, 9H, aromatic-H+ 2NH2), 6.69 (s, 1H, C5'-H), 8.48 (s, 1H, C5-H); Ms: m/z 297 (4.3), 296 (10.1), 295 (46.2), 237 (32.5), 171 (12.8), 119 (10.4), 92 (24.5), 91 (92), 90 (14.8), 77 (100), 66 (19.6), 65 (37.9), 64 (94.5), 63 (29.3), 52 (21.4), 51 (58.9). Anal. Calc. for C13H11SN7: C, 52.52; H, 3.70; N, 32.29. Found: C, 52.36; H, 3.82; N, 33.06%.
2-Amino-4-(2-phenyl-2H-1.2.3-triazol-4-yl)-nicotinonitrile (6): (0.22 g, 63%); m.p. 190˚C - 191˚C; IR: νmax/cm−1 3383 (NH), 2219 (
) and 1626 (C=N); NMR: δH(DMSO-d6) 6.87 (s, 2H, NH2), 7.25 (d, 1H, J5.6 = 8.2 Hz, C5'-H), 7.45 - 8.06 (m, 5H, aromatic −H), 7.89 (d, 1H, J6,5 = 9.2 Hz,. C6'-H), 8.65 (s, 1H, C5-H). Anal. Calc. for C14H10N6: C, 64.12; H, 3.82; N, 32.06. Found: C, 64.36; H, 3.58; N, 31.96%.
4-Amino-2-hydrazinyl-6-(2-phenyl-2H-1,2,3-triazol-4-yl)-6H-1,3-thiazine-5-carbonitrile (7): (0.3 g; 64%); m.p. 179˚C - 181˚C; IR: νmax/cm−1 3202 (NH), 2241 (
) and 1642 (C=N); NMR: δH(DMSO-d6) 7.41 - 8.01 (m, 5H, aromatic −H), 8.13 (s, 2H, NH), 8.20 (s, 1H, C6'-H), 8.45 (s, 2H, NH2), 8.60 (s, 1H, C5-H), 11.69 (s, 1H, NH). MS: m/z (%) 313 (86), 265 (16), 235 (66), 206 (36), 169 (31), 143 (18), 119 (17), 92 (21), 77 (100). Anal. Calc. for C13H12SN8: C, 49.99; H, 3.87; N, 35.87. Found: C, 50.01; H, 3.58; N, 35.96%.
2-Amino-4-(2-phenyl-2H-1,2,3-triazol-4-yl)-4,5,6,7-tetrahydrocyclopenta[b]pyran-3-carbonitrile (8): (0.3 g; 63%) m.p. >270˚C; IR: νmax/cm−1 3365 (NH), 2236 (
) and 1625 (C=N); NMR: δH(DMSO-d6) 3.13 (m, 6H, 3CH2), 6.69 (s, 2H, exchangeable NH2), 7.45 - 8.01 (m, 5H, aromatic-H), 8.42 (s, 1H, C4'-H), 8.57 (s, 1H, C5-H). Anal. Calc. for C17H15N5O: C, 66.87; H, 4.95; N, 22.94. Found: C, 66.70; H, 4.80; N, 22.80%.
4-Carboxaldehyde-2-(phenyl-2H-1,2,3-triazole hydrazone (9): A solution of (1 g, 5.78 mmole) in ethanol (20 ml) was treated with hydrazine hydrate (2 ml) and acetic acid (1 ml) was heated under reflux for 2 h, concentrated and left to cool. The solid was filtered off, washed with ethanol and dried (0.65 g, 65%). It was recrystallized from ethanol as colorless needles, m.p. 115˚C - 116˚C; IR: νmax/cm−1 3218 (NH) and 1600 (C=N). Anal. Calc. for C9H9N5: C, 57.74; H, 4.85; N, 37.41. Found: C, 57.60; H, 4.70; N, 37.80%.
1,2-Bis[(2-phenyl-2H-1,2,3-triazol-4-yl)]methylene hydrazine (10): A solution of 9 (1 g, 5.35 mmol) was treated with 4-carboxaldehyde-2-phenyl-2H-1,2,3-triazole (1 g; 5.78 mmol), boiled under reflux for 3 h, and left to cool. The solid was filtered off and washed with ethanol (yield 0.8 g, 60%). It was reerystallized from ethanol colorless needles, m.p. 218˚C - 220˚C; IR: νmax/cm−1 1600 (C=N) Anal. Calc. for C18H14N8: C, 63.15; H, 4.12; Found: C, 62.89; H, 4.53%.
1-phenyl-3-[(2-phenyl-2H-1,2,3-triazol-4-yl)methylene hydrazononomethyl]-4-(2-phenyl-hydrazono)- 1H-pyrazol-5(4H)-one (11). A solution of 9 (1 g; 5.78 mmol) in ethanol was treated with 3-carboxaldehyde- 1-phenyl-4,5-pyrazolinedione-4-(phenylhydrazine) (2 g; 6.53 mmol) was heated under reflux for 3 h, and left to cool. The solid filtered off, washed with ethanol and dried (1.1 g; 55%). It was recrystallized from ethanol in orange needles, m.p. 176˚C - 177˚C; IR: νmax/cm−1 3200 (NH), 1659 (OCN) and 1595 (C=N). Anal. Calc. for C25H19N9O: C, 65.07; H, 4.15. Found: C, 65.53; H, 4.31%.