The Effects of TiO2 Particle Size on the Properties of PTFE/TiO2 Composites ()
1. Introduction
Ceramic powder filled polytetrafluoroethylene (PTFE) has been widely used in manufacturing microwave devices because of its very low dielectric constant and excellent chemical resistance. PTFE is a kind of high performance thermoplastic polymer with unique electrical properties. It has a low dielectric constant (εr = 2.1) and an extremely low dielectric loss (tanδ = 0.0003) which are stable over a wide range of frequencies [1]. However, disadvantages of PTFE are its high linear coefficient of thermal expansion (CTE = 109 ppm∙˚C−1) and low mechanical strength. One of the efforts to control the CTE and mechanical strength of PTFE was to add inorganic fillers such as micro-fiberglass and ceramic particles into the PTFE matrix [2] [3] [4] [5]. In previous work, several researches have studied the effects of TiO2 filler content in PTFE composite, such as Rajesh [6] and Yuan [7], etc.
The object of this work is to study the effect of TiO2 particle size on the properties of TiO2-filled PTFE composites material, such as microstructure, density, moisture absorption, thermal conductivity and microwave dielectric properties. TiO2 powders were prepared by the solid state ceramic route. The different size of TiO2 powders were obtained by controlling the time of ball milling. It is expected that this work may be useful in the applications of TiO2 filled PTFE composites in practice.
2. Experimental
2.1. Materials
The raw materials used were rutile TiO2 powder and PTFE aqueous dispersion (TE-3865C, Dupont, USA). Phenyltrimethoxysilane (PTMS, TCI Corporation, Japan) was used as coupling agent. The different size of rutile TiO2 powder was ball milled for different hours and sintered at 1350˚C for 2 hours. The values of D50 (5 μm, 6.5 μm, 8 μm, 9.6 μm, 11 μm) were measured by Laser particle size analyzer. Table 1 gives the properties of PTFE and TiO2.
2.2. Fabrication of the composite
2.2.1. Coating a Coupling Agent on the Surface of TiO2 Powder
PTMS was used as coupling agent to coat the surface of filler particles. First, PTMS was hydrolyzed in alcohol and ion-free water at 55˚C for 1 h. The amount of PTMS was 1.5% of the weight of TiO2 filler and the amount of water was controlled exactly for the hydrolysis of PTMS. Then TiO2 was added and dispersed into the hydrolyzed coupling agent solution by heavy stirring for 2 h. The product was further dried in an oven at 120˚C for 3 h and silane coupling agent treated ceramic powder was obtained.
2.2.2. Preparation of PTFE/TiO2 Organic-Inorganic Composites
The TiO2 powder previously obtained was weight accurately to prepare the composites according to the weight radio TiO2/PTFE. The coupling agent treat- ed TiO2 powder was added into the aqueous PTFE dispersion and mixed by heavy stirring for 3 h to obtain a fine mixture. The mixture was then dried at 120˚C for 24 h to remove water. The dried dough was smashed by a high speed
![]()
Table 1. The properties of PTFE and TiO2.
milling. The obtained composite powder was pressed into square slices (30 mm * 20 mm * 1 mm) by cold pressing. The slices were hot pressing sintered at 360˚C and 10 Mpa. Hot treatment was performed in a program controlled oven. PTFE was melted and coalesced during hot treating, and then recrystallized while cooling.
2.3. Characterization Studies
Archimedes’ principle was used to study the density of the PTFE/TiO2 composites. The microstructure of the substrate was observed by scanning electron microscopy (SEM, model JEOL JSM-6490). Water absorption of the composite sample was measured as reported earlier by Murali etc [2]. Thermal conductivity was investigated by LFA 447 Nanoflash.
The dielectric constant (εr) and dielectric loss (tanδ) of TiO2-filled PTFE composites were measured by stripline resonator method using Agilent E8363A microwave network analyzer according to IPC-TM-650 2.5.5.5 specification [8]. The testing samples sizes were 30 mm long, 20 mm wide and 1 mm thick. The testing frequencies varied from 7.0 GHz to 13.0 GHz. The εr and tanδ of the PTFE/TiO2 composites reported in this paper was at a frequency about 10 GHz.
3. Results and Discussion
3.1. Density Tests
The dielectric property, tensile strength and thermal expansion of the substrate composites were significantly influenced by the density property. The variation of density of substrate composites with respect to TiO2 particle size is shown in Figure 1. As the D50 increased from 5 μm to 11 μm, the relative density of the substrate displayed a continuously increase. Obviously, with the TiO2 particle size increased to 11 μm, the density reached the maximum value of 2.80g/cm3. The main reasons for this are the ceramic powders trend to agglomerate with the
![]()
Figure 1. Influence of TiO2 particle size on density of PTFE/TiO2 composites.
decrease of TiO2 particle size, and the bad dispersion in the PTFE matrix which produces more pores in the matrix. However, as the D50 decreases 11 μm to 5 μm, it is more difficult for PTFE to have a good adhesion with TiO2 fillers so that many pores appeared which results in the decline of density [9].
3.2. Porosity Factor and Water Absorption
Porosity factor of the composite samples was determined by the volume fraction of inner pore using the equation:
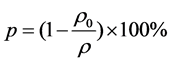 (1)
(1)
where ρ is theoretical density and ρ0 is the experimental density.
Density of the composite samples had been calculated using the rule of mixtures:
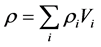 (2)
(2)
 (3)
(3)
where ρi, vi and mi are the density, volume fraction and weight of the filler and matrix, respectively.
Previous studies showed that the electric property of substrate composite was influenced by moisture absorption, significantly. Since water has high loss tangent, the electrical property would deteriorate while water absorption increases. As the weight fraction of TiO2 and PTFE were fixed, the water absorption was general determined by porosity factor. For smaller ceramic particle size, agglomeration of ceramic filler leads to increasing fraction of porosity and water absorption. The variation of porosity factor and water absorption of the composites as a function of TiO2 particle size was shown in Figure 2. The porosity factor had shown a decreasing trend with ceramic particle size increasing. The water absorption had the same regular pattern with porosity factor, and reached the minimum value of 0.04% when D50 = 11 μm. This phenomenon maybe due to that porosity factor increases with increasing filler particle size, which promotes water absorption of the substrate.
3.3. Morphology Aspects
The cross sectional SEM images of TiO2/PTFE composites are shown in Figures 3(a)-(e). The filler particle size changed from 5 μm to 11 μm in successive. It can be seen that all samples exhibit two-phase structure viz. PTFE and ceramic powder. Since PTFE has a low surface energy, it is difficult for ceramic powders to have a good adhesion with PTFE. Many pores can be observed in all images. As particle size increases, a denser microstructure with lesser pores is observed. Sample showed well-dense and homogeneous microstructure when D50 = 11 μm. The typical planar SEM micrograph of D50 = 11 μm is shown in
![]()
Figure 2. Variation of water absorption and porosity factor with respect to TiO2 particle size.
![]()
Figure 3. Cross sectional SEM images of TiO2 filled PTFE composites (a) D50 = 5 μm; (b) D50 = 6.5 μm; (c) D50 = 8 μm; (d) D50 = 9.6 μm; (e) D50 =11 μm; And surface SEM images of the composites when D50 = 11 μm.
Figure 3(f). It can be seen that the surface of the substrate is coated by PTFE and no ceramic powders are observed.
3.4. Thermal Conductivity
The variation of thermal conductivity of the composites as a function of D50 is shown in Figure 5. The thermal conductivity displayed a decline at first, and then increased slowly after λ dropping to the minimum value of 0.38 W(m℃)−1. The surface area of ceramic powder increased with the decline of D50, which result in the sharply increasing of interface region. Since more interface region means worse contact between ceramic powder and PTFE, this region would deteriorate the thermal conductivity. However, the main reason why λ has an increase when D50 = 5 μm is that the agglomeration of ceramic filler leads to in
![]()
Figure 4. Variation of thermal conductivity with respect to TiO2 particle size.
![]()
Figure 5. Variation of dielectric constant and loss tangent at 10 GHz of TiO2 filled PTFE composites with respect to the value of D50.
creasing fraction of porosity and high humidity which would promote the value of λ when D50 = 5 μm.
3.5. Dielectric Properties
The dielectric properties of the substrate composites depend on not only the dielectric properties of the components but also other factors such as the size of fillers, the interactions between ceramics and polymers, and the dispersion of fillers in polymer matrix [9] [10] [11] [12] [13]. Figure 5 shows the dielectric constant and loss tangent of the composites as a function of D50 of TiO2 particle size. The composite has low loss tangent values (<0.002). As the particle size increases, the loss tangent declines sharply and reaches the minimum values of 0.0012 when D50 = 11 μm. As we know, water has unfavorable dielectric properties (εr = 70, tanδ = 35) [3], high water absorption could deteriorate the dielectric property. The relative dielectric constant of the composites show a sharp decreasing trend at first, and then a slight increase after dropping to the minimum value when D50 = 6.5 μm. The changing of relative permittivity was opposite to that of loss tangent in a manner, since relative densities increase with D50 values growing. The main reason for the εr catch the peak value of 6.9 is the high water absorption has promoted the dielectric constant.
4. Conclusion
Rutile TiO2 powder was modified and filled in PTFE matrix in 50wt% through cold pressing and hot treating process, and to fabricate TiO2/PTFE composites for microwave substrate applications. Microstructure, density, moisture absorption, thermal conductivity and microwave dielectric properties of PTFE/TiO2 composites were studied from D50 = 5 μm to 11 μm filler average particle size. The moisture absorption and porosity factor decrease, whereas the density increases as the D50 of ceramic particle size increase from 5 μm to 11 μm. The loss tangent shows a decreasing trend with the D50 increasing. The dielectric constant sharply drops up to 5.6 when D50 = 6.5 μm and then slightly increases. The changing of thermal conductivity was same to that of dielectric constant in a manner. The composite has a dielectric constant of 6.8, a loss tangent of 0.0012, moisture absorption of 0.04%, density of 2.797 g/cm3, and thermal conductivity of 0.233W(m˚C)−1. Present study shows that TiO2 filled PTFE composites are promising candidates for microwave circuit applications.