Effect of Dye Structure on the Photodegradation Kinetic Using TiO2 Nanoparticles ()
1. Introduction
Dyes decomposition from textile wastewater is recognized as an important environmental treatment due to their potential toxicity and coloration [1] . The characteristics of these dye compounds have generated some challenging environmental problems, such as reducing the percentage of light penetrating into the water which reduces photosynthetic activity in aquatic media. A low concentration of visible dye is intolerable and can cause a health hazard for humans [2] . Various physical, chemical and biological methods such as adsorption, photolysis, chemical precipitation, chemical oxidation and reduction, electrochemical precipitation have been employed for the removal of dyes from wastewater [3] [4] [5] [6] .
Among these approaches, photodegradation is considered as a promising technology because it is a versatile, low-cost and environmentally benign treatment for most of pollutants. They are based on the generation of the strongly oxidizing hydroxyl radicals (OH・). These radicals are characterized by a little selectivity of attack, and are able to oxidize a variety of organic compounds that could be present in water and wastewater, due to their high oxidative capacity (redox potential of OH: E0 = 2.8 V), yielding CO2 and inorganic ions [7] .
Advanced oxidation processes (AOPs) are based on the photogeneration of valence band holes (h+) and conduction band electrons (e?) which react with water and oxygen to form highly reactive species such as OH・, H2O2 and O2・? on the semiconductors surface. These prepared species is strong enough to break the different bonds in the organic pollutants. Hence, AOPs are effective and rapid methods to remove toxic and bio-refractory pollutants such as dyes in wastewaters [8] .
With respect to other photocatalysts, TiO2 has so far been considered as one of the most preferred materials due to its non-toxicity, resource availability, low-cost and high stability [9] . It is capable of decomposing a wide variety of organics and inorganics in both liquid and gas phases, when its aqueous form is irradiated with light energy greater than its band gap energy (Eg = 3.2 ev) [10] .
Generally the photodegradation rate is strongly related to the adsorption affinity of dyes on the catalyst surface [11] . The affinity between the catalyst TiO2 and pollutants is an important factor in enhancing the photodegradation efficiency [12] .
A few research projects focused on the relation between the chemical structure of dyes molecules and its resistance to the photocatalytic degradation [13] [14] [15] .
In the present study, the effect of chemical structure of organic dyes on the photodegradation rate was investigated using TiO2 catalyst. Methyl orange (MO) and Methyl Orange (BBT) were chosen as model organic pollutants since they contain different functional groups. We are going to discuss the effect of pH on their adsorption and degradation, to compare the rate constant of degradation of each dye according to their chemical structure and stability. The molecular structures of MO and BBT in basic and acidic medium are shown in Figure 1, where their Pka values are respectively 3.4 and 7.1.
2. Materials and Methods
2.1. Materials
Titanium dioxide powder P25 was employed since it remained the state of the art photocatalyst [16] [17] . It was supplied by Evonik-Degussa. The particle diameter, estimated by transmission electron microscopy (TEM), was approximately equal to 25 - 30 nm.
![]()
Figure 1. Chemical structure of Methyl Orange and Bromothymol Blue.
2.2. Surface Characterization
The chemical and surface properties of the solid samples were characterized by, Fourier transform infrared spectroscopy (FTIR), X ray diffraction and electrophoretic mobility.
The kinetic study was followed by measuring the absorbance in visible for the colored dyes. Fourier transform infrared spectroscopy was used to analyze the surface chemistry of the powders. Diffuse reflectance (DRIFT) spectra were recorded using a Fourier transform infrared spectrometer (Bruker IFS 55). FTIR experiments were also carried out in transmission mode by means of a Fourier transform infrared spectrometer (JASCO TF/IR 6300 type A).
UV/visible diffuse reflectance spectra were obtained using a UV/vis spectrophotometer (Shimadzu).
Electrophoretic mobilities were measured with Zetaphorometer ZIII from SEPHY-CAD society instrument. The solutions for zeta potential measurements contained 10 mg/L of TiO2 dispersed in 10−3 M NaCl aqueous solution. Prior to each measurement, the suspensions were sonicated for 2 min.
The phase structure of the TiO2-P25 photocatalyst was examined by X-ray diffraction (XRD) using X-ray diffractometer with Cu Kα radiation (λ = 0.15406 nm).
2.3. Photodegradation Experiment
Experiments were carried out, at room temperature, in a glass Petri dish (diameter 5.8 cm) reactor with 80 mL capacity placed on a magnetic stirrer. The light source was provided by a mercury lamp positioned outside the reactor and parallel to it, emitting in the near-UV. The incident light intensity reaching the top of the reacting suspension was equal to 10 - 6 Einstein L−1∙s−1.
The catalyst was mixed with the pollutant aqueous solution (Methyl Orange or Bromothymol Blue) under stirring. For all experiments, the photocatalyst content and the initial pollutant concentration were equal to 1 g/L and 10 mg/L, respectively. According to the pKa values and in aims to obtain a homogenous structure of each dye (conjugate acid or base), the initial pH was adjusted to the desired value (pH 3 or 10) through NaOH or HCl addition. At the beginning of each experiment, the suspension containing the pollutant was magnetically stirred in the dark for 1 h to reach the adsorption/desorption equilibrium [18] . After this, the solution was then irradiated using UV light under continuous magnetic stirring. At different times, 3 mL of the suspension were withdrawn with a glass syringe and the catalyst particles were separated from the liquid by filtration (0.1 nm PTFE Millipore filter). A sample was taken at the end of the dark adsorption period (irradiation time = 0). The pollutant concentrations were analyzed by a UV/vis spectrophotometer. For Methyl Orange, the absorbance value in acidic medium is at the wavelength of 464 nm while at basic medium is 520 nm [19] [20] [21] . For Bromothymol Blue, the absorbance value in acidic medium is at the wavelength of 428 nm while at basic medium is 616 nm [22] .
It appeared important to perform experiments in the absence of direct photolysis in order to gain information about the photocatalytic process. Under UV light irradiation but in the absence of titania, the results indicated no degradation of the pollutants (Methyl Orange or Bromothymol Blue). The degradation remained also negligible in the absence of UV light (but in the presence of TiO2). A significant degradation of the contaminants occurred only in the simultaneous presence of titania and UV light.
3. Results and Discussion
3.1. Characterization of Titanium Dioxide
Figure 2 displays the XRD pattern of the TiO2 P25 particles. The curve shows typical pattern of the anatase powder of good quality (good crystallinity). The peaks located at 25.4, 37.8, 48.0 and 54.5 respond to the (101), (004), (200), (105 and 211) planes of the anatase phase (JCPDS 21-1272), and that located at 27.5, 36.1, 54.4 respond to the (110), (101), (211) planes of the rutile phase (JCPDS 21-1276), respectively.
The FTIR is mainly used to analyze the TiO2 surface. Figure 3 displays the DRIFT spectra of bare TiO2. The analyses were performed at atmospheric pressure, the spectra of titania powders showing a broadband in the 3800 - 2700 cm−1 region (peak around 3400 cm−1) with an emergent peak at 3692 cm−1, three bands at 1620, and 1420 cm−1, and a large band at around 670 cm−1. The broad vibration band between 3650 and 2600 cm−1 is associated with the OH stretching mode of water molecules (physisorbed molecular water), while that at 1620 cm−1
![]()
Figure 2. X-ray diffraction pattern of TiO2 P25; * Anatase, × Rutile.
![]()
Figure 3. Infra red transmission spectra of TiO2 P25.
is assigned to their bending modes. According to various studies carried out to associate the IR bands to specific groups adsorbed on the oxide surfaces [23] [24] the bands in the 3800 - 3600 cm−1 region are due to the stretching modes of different types of free hydroxyl groups adsorbed on the crystalline surfaces of titania or on defects located on specific surfaces [24] [25] . The peak at 3692 cm−1 is assigned to hydroxyl groups chemisorbed on the defects of specific crystal faces of anatase. The bands in the range 1600 - 1300 cm−1 are generally associated with carbonate groups; thus, the bands at ∼1420 cm−1 are assigned to carbonate species formed by the adsorption of atmospheric CO2 [26] [27] . Absorption in the range 1000 - 600 cm−1 is due to the surface vibrations of the Ti-O bonds.
Zeta potential measurements were used to quantify the conditions leading to the stability of TiO2 dispersions as well as the surface charge. The variation of the zeta potential is shown in Figure 4.
The pH variation from 3 to 10 was accompanied with a variation of zeta potential between +35 mV in acidic medium to around −45 mV in basic medium. The isoelectric point of TiO2 nanoparticles was pH 6.2, which was similarly reported by Zhao et al. [28] . Rather than stability of our TiO2 nanoparticles in basic medium these results reveals the positive surface charge of TiO2 in acidic medium (pH < 5.2) and negative charge in basic medium (pH > 7.2).
3.2. Adsorption Study over TiO2 Surface
The adsorption of Methyl Orange over TiO2 surface was studied at different pH values. Figure 5 shows the adsorption capacity Qe of MO as a function of equilibrium concentration Ce at pH = 3, and 10.
All adsorption isotherms presents an increasing shape, (increasing of quantity adsorbed in mg per g of adsorbent), which decreases gradually to reach a plateau as a function of Ce (Concentration of adsorbate at equilibrium).
The highest quantity of Methyl Orange adsorbed was observed at pH 3, which is Qe max = 36.4 mg/g followed by pH 10 for Qe = 13.8 mg/g and the lowest
![]()
Figure 4. Zeta potential of TiO2 P25 versus pH.
![]()
Figure 5. Adsorption isotherm of Methyl Orange on TiO2 P25 at different pH.
amount was for pH 7, Qe = 7.2 mg/g of TiO2.
At pH 3, where pH < PZC TiO2, they are mainly present in the forms of Ti- OH and/or Ti-OH2+, resulting in the formation of hydrogen bonds with MO molecules. In addition, MO is a polar molecule, and it could be adsorbed on the surface of TiO2 via van der Waals force. On the other hand, O, N and S atoms in MO could form hydrogen bonds due to their high electronegativity with the surface hydroxyl groups of TiO2. Therefore at pH 3, we think that both van der Waals force and hydrogen bond formation are contributive to MO adsorption [29] .
At pH 10, where pH > PZC, the surface hydroxyl groups of TiO2 will be dissociated to form TiO−; thus electrostatic repulsion between negatively charged MO molecules and TiO− is obtained, leading to lower adsorption capacity. Then the adsorbed percentage for MO in the low pH region is greater than in the high pH region.
Figure 6 represents the adsorption isotherm of BTB at pH 10 and 3. Both isotherms show an ascending shape. The isotherm of basic medium similar to S shape with a higher adsorbed quantity which close 40 mg/g of TiO2 against approximately 25 mg/g in acidic medium.
At pH 3, the BTB molecule with an apparent neutral charge is adsorbed directly on TiOH2+ surface by hydrogen bonding. While at pH 10 the BTB starts with a very low adsorption amount due to the repulsion between the negative charged BTB and TiO2− surface. After 40 mg/L of equilibrium concentration the adsorbed quantity increased rapidly where the sharp change in the slop reflect the variation in the nature of the surface and enhances the proposition of the formation of multilayer of Bromothymol Blue by van der Waals forces.
Langmuir and Freundlich adsorption models, two well-known models, were employed to the analysis of our isotherm at pH 3. For this, the following Equations (1) and (2) were used for Langmuir and Freundlich adsorption model studies, respectively [30] [31] .
![]()
Figure 6. Adsorption isotherm of Bromothymol Blue on TiO2 P25 at different pH.
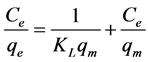 (1)
(1)
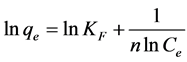 (2)
(2)
where Ce is the equilibrium concentration of adsorbate, qe is the equilibrium adsorption capacity, KL is the Langmuir adsorption equilibrium constant, qm is the maximum adsorption capacity, KF is the Freundlich adsorption equilibrium constant, and n is the constant of Freundlich adsorption. The Langmuir and Freundlich adsorption isotherms that are obtained using the above two equations are given in Figure 7.
As seen in the figure, good linear correlation is obtained for both adsorbate MO and BTB, indicating that our experimental data fit well with Langmuir adsorption model. In the case of Methyl Orange the correlation coefficient (R2) for Langmuir and Freundlich adsorption isotherm were 0.9987 and 0.9354, respectively. This indicates that the experimental data has a better fit with the Langmuir adsorption model. As discussed above, MO is adsorbed on the TiO2 surface by electrostatic attraction at pH 3. Thus, the homogeneous nature of surface hydroxyl groups led to Langmuir type adsorption. This implies that the adsorbed layer is monolayer coverage.
In the case of Bromothymol Blue the correlation (R2) for Langmuir and Freundlich adsorption isotherm were 0.9921 and 0.9047, respectively, which also indicate that the experimental data has a better fit with the Langmuir adsorption model. For the Bromothymol Blue, the only factor that contributes to the adsorption is the hydrogen bond Vs a homogenous positive charged hydroxyl group on the surface of TiO2. This implies also that the adsorbed layer is monolayer coverage.
To compare the affinity of different adsorbates to the titane sorbent, the Langmuir isotherm parameters were investigated. Table 1 shows the value of Langmuir isotherm for Methyl Orange and Bromothymol Blue at pH 3. As the results shown the values of constant b show a very higher affinity of MeO toward titan surface and this may due to van der Waals attraction between them, at pH 3.
![]()
![]() (a) (b)
(a) (b)
Figure 7. Langmuir adsorption isotherms (a) and Freundlich adsorption isotherms (b) for Methyl Orange and Bromothymol Blue at pH 3.
![]()
Table 1. Langmuir isotherm constant for Methyl Orange and Bromothymol Blue.
3.3. Photocatalytic Degradation and Kinetic Study
The degradation experiments by UV irradiation are expected to follow the pseudo-first-order kinetics with respect to the dye concentration in the bulk solution. Therefore the kinetics of the study can be analyzed by the Langmuir? Hinshelwood model [32] [33] :
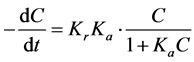 (3)
(3)
where 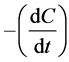 is the photocatalytic oxidation rate, Kr is the photocatalytic
is the photocatalytic oxidation rate, Kr is the photocatalytic
oxidation rate constant, Ka is the adsorption equilibrium constant, and t is the reaction time. If the product of Ka and C is significantly smaller than 1, the above equation can be simplified to a first-order reaction:
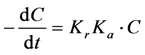 (4)
(4)
Consequently an integrated form of Equation (4) can be represented as follows:
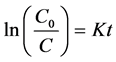 (5)
(5)
where K = KrKa is the apparent rate constant in min−1. The kapp value can be obtained by plotting ln(C0/C) vs. t.
Since the pH is a main factor that contributes the photodegradation of dyes on titanium dioxide [34] , the photodegradation of Methyl Orange and Bromothymol Blue was studied as a function of pH, acidic and basic medium.
Methyl Orange
The photocatalytic degradation of Methyl Orange in acidic and basic medium (pH 3 and 10) are illustrated in Figure 8.
The two curves show a similar trend, the concentration of Methyl Orange decrease as time increase in acidic and basic medium. The data at irradiation 0 corresponds to the remaining pollutant concentration after one hour of stirring in the dark. Consequently, it gives access to adsorption capability which is previously studied with the adsorption isotherm.
A weak adsorption of Methyl Orange on TiO2 surface at pH 10 are observed since there is a very slight diminution of the Methyl Orange concentration after
![]()
Figure 8. Photocatalytic degradation of Methyl Orange at pH 3 and pH 10 as a function of the time of irradiation for the Titanium P25.
60 min of dark period. On the other hand, in acidic medium (pH 3), the high diminution of the concentration of Methyl Orange at t = 0, reflect the high adsorption amount of Methyl Orange molecules on titane surface at this pH.
These results are consistent with those showed by the adsorption isotherm of Methyl Orange presented in Figure 5, where the quantity of Methyl Orange adsorbed at pH 3 is much higher than those at pH 10.
Based on the zeta potential variation of Titanium dioxide as a function of pH, and the pKa of Methyl Orange, at pH 10 all the species bear a negative charge. The titanium dioxide is negatively charged (zeta potential of ca. −40 mV see Figure 4) while the acidity constant of Methyl Orange is equal to 3.75. The small value of the pollutants adsorbed amounts can be explained by the electrostatic repulsions between the pollutant and the catalysts.
In kinetic study, Methyl Orange photodegradation proceeds more favorably at pH 3 [20] . The degradation of Methyl Orange occurs after 60 mins. The difference in the photocatalytic activity can be related to the difference in adsorption amount between two pHs [25] .
The photodegradation kinetic of Methyl Orange was then investigated with the results shown in Figure 10. This figure represents the evolution of Ln (C0/C) as a function of time. The linear evolution confirms that the degradation of Methyl Orange by titanium dioxide follow the pseudo-first order.
The calculated the pseudo-first-order rate constant K1 for pH 3 was 0.07, and K2 0.02 for pH 10.
Bromothymol Blue
Figure 9 represents the photocatalytic degradation of Bromothymol Blue at pH 3 and pH 10.
The BTB concentrations (C0) of pH 3 and pH 10 after the dark period are close to the initial one (10 mg/L). This indicates a low adsorbed amount of Bromothymol Blue on the catalyst surface during the dark period.
This behavior can be verified from the adsorption isotherm that shows a low
![]()
Figure 9. Photocatalytic degradation of Bromothymol Blue at pH 3 and pH 10 as a function of the time of irradiation for the Titanium P25.
adsorption amount on the TiO2 surface for low concentrations (see Figure 6).
In this figure each curve shows a similar trend. The bulk concentration of Bromothymol Blue decreases as time increases. The photocatalytic performance is affected by the pH of the solution. In basic medium (pH 10), the Bromothymol Blue solution is almost fully degraded within 70 min under UV light. This time increases for acidic medium.
In order to investigate the kinetic parameters, a plot of Ln (C0/C) versus the irradiation time (t) is reported in Figure 10.
The linear evolution confirms that the degradation of Methyl Orange by titanium dioxide follow the pseudo-first order.
The calculated the pseudo-first-order rate constant K1 for pH 3 was 0.01, and K2 0.04 for pH 10.
Table 2 shows the different kinetic parameters and the adsorption percentage of Methyl Orange and Bromothymol Blue in acidic and basic medium.
In acidic medium the adsorption percentage of MO on TiO2 surface is 76% which is much higher than that of BTB at the same pH. This result was also verified with the constant value of Langmuir isotherm, where the constant b shows a very high affinity of MO toward titan surface relative to that of BTB. In the same context the value of kinetic constant of MO at pH 3 is too much bigger than that of BTB at the same pH, which indicate that the adsorption on the surface is the main factor that affect the degradation of MO and BTB on the titane surface at pH 3 [34] .
In acidic medium the adsorption percentage of MO on TiO2 surface is 76% which is much higher than that of BTB at the same pH. This result was also verified with the constant value of Langmuir isotherm, where the constant b shows a very high affinity of MO toward titan surface relative to that of BTB. In the same context the value of kinetic constant of MO at pH 3 is too much bigger than that of BTB at the same pH, which indicate that the adsorption on the surface is the
![]()
Figure 10. Pseudo-first kinetics of Methyl Orange and Bromothymol Blue at pH 3 and pH 10.
![]()
Table 2. Kinetic parameters and adsorption percentages of Methyl Orange and Bromothymol Blue in acidic and basic medium.
main factor that affect the degradation of MO and BTB on the titane surface at pH 3 [34] .
In basic medium the adsorption percentage of MO and BTB are considered equal and negligible, due to the repulsion between dye molecule and titania surface as we explained before. If we compare the kinetic constant of MO and BTB, we noticed that the degradation of BTB occurs two times faster than that of MO. These results reveal that the adsorption on the surface is not the only factor that affects the photodegradation of organic molecules.
The variation of chemical structure of the molecule as a function of pH can vary the bond energy of its structure [36] and consequently its stability. The efficiency of OH radical liberated from the bulk of TiO2 can be affected by the stability of structure [37] . However, in basic medium the BB-structure of BTB is negatively charged on its SO3 function group, with 3 rings and 1 hydroxyl group, two bromines and ketone group. On the other hand, Methyl Orange has an azo ion structure in basic medium which is contain two rings connected by nitrogen group, and negative charge on SO3 function.
4. Conclusions
In this paper we compare the adsorption affinity of Methyl Orange and Bromothymol Blue on TiO2 P25 nanoparticles on acidic and basic medium. The nanoparticles are characterized by XRD, and Zetaphorometer, the UV/visible spectroscopie was used to follow the variation of the concentration of dyes during photodegradation.
The adsorption isotherm was performed at two pHs (3 and 10), then the photodegradation process under UV light was compared for the two dyes toward titania nanoparticles at different pHs.
In acidic medium, the adsorption was the main factor that affects the photodegradation where the rate constant of the degradation was compatible with the adsorption percentage. In basic medium, the chemical structure of organic molecule changes due to variation of pH, which reflected by a dramatic decreasing of adsorption percentage, and for the same values of adsorption percentage between MO and BBT we noticed a big differences in the rate constant of degradation process, this result indicates that the adsorption was no more the main factor that affects the photodegradation of molecules. The chemical structure of the molecule becomes effective at this stage and may play an essential role in the efficiency of OH radical for the degradation of organic molecule.
Acknowledgements
The author is gratefully acknowledge the support of this research by the “The Research Laboratory of Materials, Catalysis, Environment and Analytical Methods (MCEMA)” under the supervision of Prof. Joumana Toufaily and Tayssir Hamieh.