1. Introduction
Conjugated linoleic acid (CLA) has been found to be an extraordinary essential fatty acid with miscellaneous functional effects on the human body. The most beneficial CLA isomers participate with biological performance are 9c11t-C18:2 and 10t12c-C18:2 [1] [2] .
Despite CLA exists at levels of 0.3% - 0.8% (w/w) of the fat in beef and dairy products of the ruminants, this negligible level cannot provide the recommen- ded 3 - 3.4 g of CLA per day that is necessary to produce the desired physiological effects [1] . Furthermore, the high consumption of such natural resources is not recommended due to intake of undesirable amounts of saturated fats and cholesterol. For this reason, an alternative source that contains high amounts of CLA that is low in saturated fat and cholesterol would be recommended for the human diet [3] . CLA commercialization has been highly studied through various methods and with regard to CLA insufficient daily intake by the natural source. The most common way to produce CLA is base-catalyzed isomerization [4] [5] . However, the severe conditions (e.g., high temperature, solvents and large amount of alkalis) which are usually required for the routine isomerization were to lead to the formation of unwanted trans isomers, polymerization and cyclization reactions and thus, the reduction of theoretically expected yield [6] .
In order to produce commercial CLA with less unwanted isomers, Abney and Anderson (2002) applied linoleic acid methyl esters (LAMEs) as the substrate of isomerization which was found to result in the production of a high yield of conjugated linoleic acid methyl esters (CLAMEs). They proposed the isomerization of LAMEs by using negligible amounts of alkali catalyst, at a low temperature and in the presence of phase transfer catalyst (PTC) instead of solvents. Their technique resulted in increasing the degree of the isomerization from 6% without PTC to 90% [4] . Despite this improvement, its limitation is that it was based on the traditional 1-factor-at-a-time approach; and thus, time-consuming and also the fact that it was almost impossible to achieve real optimal condition. Furthermore, it has been demonstrated that the results of one-factor-at-a-time experiments, often ignore the interactions between factors that are present concurrently [7] . In this research, high linoleic sunflower oil which contains more than 65% linoleic acid (LA), a fatty acid with the potential to be isomerized to CLA, and could therefore be utilized to produce CLA-rich oil was applied as the isomerization substrate. A detailed study is conducted on the optimization of the isomerization using response surface methodology (RSM) with the aim of improving the degree of isomerization and thus the process yield compared to previous researches.
2. Experimental Procedure
2.1. Materials
High linoleic sunflower oil (>65% linoleic acid) fatty acid methyl esters (FAMEs) were prepared as described previously [8] . PEG400, NaOCH3, H3PO4 (85%; w/w), urea, the standard mixture of 37 fatty acid methyl esters (external standard, dissolved in hexane), CLA methyl ester standard mixture cis-9, trans-11 and trans-10, cis-12 isomers (>98% pure), methyl-heptadecanoate (17:0, internal standard) and micro-membrane syringe filters and Whatman No.2 qualitative filter papers (Whatman Intl. Limited, Kent, UK) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Methanol, ethanol, n-hexane (GC grade), isooctane (GC grade), HCl (6 N), NaOH, anhydrous sodium sulfate and other chemicals were obtained from Fisher Scientific (Pittsburgh, PA, USA).
2.2. Methods
2.2.1. Experimental Procedure
The first phase of the experiments was the optimization of the isomerization or conversion of LAMEs from sunflower oil into CLAMEs. The second stage involves the production of enriched CLA via four sequential steps including the saponification, hydrolysis, phase separation and purification through the two- step urea inclusion crystallization. All stages were performed using a 2 litre double-walled stainless steel pressure laboratory reactor (IKA, LR 2000 P, Germany) equipped with the pressure gauge, a mechanical stirrer, water condenser, temperature regulator, sampling outlet, and an adjustable water bath providing the desired temperatures.
2.2.2. Experimental Design for RSM Study
The design of experiment (DOE), data analysis and optimization procedures were performed using the Minitab v.14 statistical package (Minitab Inc., 2000, State College, PA, USA). RSM was applied to determine the effect of four independent variables (i.e., reaction time, the temperature, the amounts of NaOCH3 and PEG400) or their interactions and on the mass of percentage of total CLAMEs (% w/w) as a response. Thirty isomerization treatments were designed based on a central composite design (CCD) considering five levels for each factor. The experimental matrix of isomerization is indicated in Table 1. The experiments randomized to minimize the effects of any extraneous factors on the actual response and method repeatability was assessed by repeating the centre point six times [9] . Isomerization was performed according to the DOE obtained from RSM (Table 1). Approximately, 500 g of distilled FAMEs of sunflower oil, NaOCH3 in the form of a paste with negligible amounts of methanol and the PEG400 were placed into the reactor. The reactor was then sealed and nitrogen was introduced as an inert gas to prevent FAMEs from oxidation (at 1.5 bar). The nitrogen was discontinued and the agitation (300 rpm) started to proceed up to the desired temperatures and based on the reaction times defined in the DOE matrix.
The reaction was then terminated with the addition of 1 mL of H3PO4, during which phosphate salts were precipitated. The reactor was cooled to 80˚C, the precipitates were removed and the contents were transferred into a separatory funnel for the phase separation [10] . The conjugated fatty acid methyl esters (CFAMEs) mixture was then washed twice with deionized water (dH2O) (80˚C - 90˚C) to separate the residues of NaOCH3, soaps and PEG400. H3PO4 was added and further washing was repeated until the pH of the drain reached to the neutral value of 7 to remove PEG400 from the fatty acid layer [11] . The layers allowed to be separated for 20 min and aqueous bottom layer was decanted. The CFAMEs from the upper layer mainly consist of CLAMEs transferred into a rotary
![]()
Table 1. Matrix of the isomerization central composite design (CCD) obtained from response surface methodology.
cCenter point.
evaporator (80˚C for 1 h) and samples were stored under nitrogen at −18˚C before any further experimental procedures.
2.2.3. Saponification
The CFAMEs (200 g, 0.679 moles) produced under the optimum condition, 200 mL water, 200 g ethanol and NaOH (38 g; 0.95 moles) were combined in the pressure lab reactor to produce sodium conjugated linoleate (CLA soap). The reactor was then sealed and nitrogen was purged )0.3 bar( to avoid the mixture from foaming under intensive stirring (300 rpm), then temperature raised and kept for 1 h at 85˚C [12] . The CLA soap was isolated from the mixture using a centrifuge (3000 rpm for 15 min) and ethanol removed from the soap (1 h at 80˚C under vacuum evaporation) prior to hydrolysis of the soap and to prevent the re-esterification reaction [6] .
2.2.4. Soap Hydrolysis
The sodium salt of the conjugated fraction was transferred into the reactor. H3PO4 was added into the mixture at 80˚C and a vigorous mixing (300 rpm) was initiated to complete the conversion of CLA salts into free fatty acids until the pH of the bottom layer reached 2 - 3 [13] . The reactor temperature was maintained at 80˚C to avoid the emulsion formation between CLA and the aqueous layer. Then aqueous layer decanted, and the temperature increased to 90˚C. To eliminate the residual soap, the organic phase was washed with distilled water (100 mL) two times and phase separation was further completed through centrifugal separation (3000 rpm for 15 min). The organic phase dried under a vacuum evaporation, cooled to the room temperature and stored at −18˚C under nitrogen in sealed stainless steel containers.
2.2.5. Urea-Inclusion Crystallization
This stage comprises a two-step urea inclusion crystallization on the free fatty acid mixture to purify the CLA. In 1st step, 1 kg of the crude CLA obtained from the previous stage was introduced into the urea-saturated methanol solution (1.5 kg/4 L) in 6 portions while mixture was stirring at 70˚C [14] . The mixture was then cooled at rate of 0.2˚C/min to reach the room temperature using a cooling unit (Frigomix1000, Sartorius Stedim Biotech, Germany). The resulting mixture was then filtered using syringe micro-membrane filters (GD/X syringe filters with a pore size of 0.45 μm, Whatman Co.) to separate the saturated and most of the monoenoic fatty acids. Hence, such impurities were filtered from the filtrate (mother liquor) in the form of crystalline urea-inclusion compounds (UICs) [15] . The filtrate consisted of unused urea and unsaturated fatty acids (e.g., CLA and alpha-linolenic acid) was transferred into a vacuum rotary, and residual methanol was evaporated until a solid product (unsaturated fatty acid mixture) obtained. Afterwards, HCl (6 N) and 1 L of dH2O were added to the solid product and the mixture stirred. The addition of HCl to the mixture resulted in an improved separation of the fatty acid and aqueous phases and the organic phase was collected using a separatory funnel [16] . The fatty acid mixture obtained from the 1st stage was again added to a urea/methanol saturated solution and the crystallization was repeated in the 2nd step. The mixture then filtered on a Buckner funnel under reduced pressure through Whatman filter papers to collect the crystalline CLA in the form of a urea inclusion compound (CLA-UIC). To isolate the urea from the CLA, 1 L of the dH2O was added into the filtrate CLA-UIC. The CLA was extracted using 1 L of hexane and washed three times using dH2O and then dried over an anhydrous sodium sulfate to remove the water [17] . The high-purity CLA was finally produced using a vacuum evaporator to remove residual hexane and then transferred to the GC section for further analysis.
2.2.6. Gas Chromatography (GC) Analysis
The composition and content of the fatty acids before and after the isomerization and purification processes were determined by GC analysis (Agilent 7890, Agilent Inc., DE, USA) equipped with flame ionization detector (FID) and autosampler injector.
The CFAMEs obtained from each isomerization treatment and the internal standard solutions were prepared following a procedure similar to what was done for the FAMEs [8] . Identification of the isomers of the CLA and the other fatty acid was performed by comparing the retention times and elution orders of the corresponding peaks with those of the external standards (i.e., mixture of cis-9, trans-11 and trans-10, cis-12-CLA methyl esters and 37 component fatty acid methyl esters mixture, Supelco, USA). The purified CLA obtained from the 1st and the 2nd stages of the urea-inclusion crystallization were methylated according to the previous method [18] . The fatty acid composition of the FAMEs, CFAMEs resulting from each treatment of the isomerization and the purified CLA obtained from the 1st and the 2nd stages of the urea-inclusion crystallization were analyzed using the GC conditions as recommended previously [19] . The samples were transferred into the amber auto-sampler vials (1.5 mL) for gas chromatography (GC) analysis.1 μL of each sample was injected to the column inlet using Agilent autosampler syringes (10 μL, part number 5181 - 1267, Agilent Technologies, Australia) at a split mode (20:1) on a SP 2560 fused silica capillary column coated with 100% cyanopropyl (100 m, 0.25 mm I.D., 0.20 μm film thickness; Supelco Inc., Bellefonte, PA). The column temperature was held at 70˚C for 4 min; then, the temperature was raised to 175˚C at the rate of 13˚C/min and was held at 175˚C for 27 min. Thereafter, the temperature was programmed to be increased at 4˚C/min to 215˚C and was held for 31 min. The inlet and FID temperatures were set at 230˚C and 250˚C, respectively. The carrier gas was the high-purity nitrogen with constant flow rate of 1 mL/min. A 100-m highly polar SP 2560 fused silica capillary column coated with 100% cyanopropyl (100 m, 0.25 mm I.D., 0.20 μm film thickness; Supelco Inc., Bellefonte, PA) was used to provide a good resolution of the fatty acids and especially the CLA isomers. The mass percentage of each FAMEs including the CFAMEs was measured following Equation (1). The total CLAMEs (TCLAMEs; % w/w) and the production yield of the total CLAMEs (YTCLAMEs; % w/w) were calculated using Equations (2) and (3).
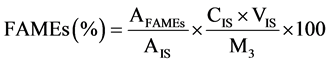 (1)
(1)
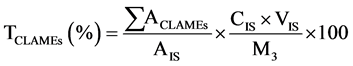 (2)
(2)
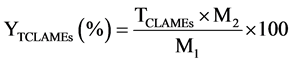 (3)
(3)
where AFAMEs and AIS represent the peak areas of the individual FAMEs and the internal standard (methyl heptadecanoate), respectively. CIS and VIS are the concentration (mg/mL) and the volume of the internal standard solution (mL), and  represents the total peak area of the CLA methyl esters. M1 (mg) is the mass of the initial sunflower oil. M3 (mg) is the mass of each sample which was taken for the GC analysis. M2 (mg) shows the mass of CFAMEs layer which was weighed after the washing and separation process for each isomerization experiment. The fatty acid composition of the samples before and after the isomerization was then identified by comparison of the peaks with retention time of the respective external standards.
represents the total peak area of the CLA methyl esters. M1 (mg) is the mass of the initial sunflower oil. M3 (mg) is the mass of each sample which was taken for the GC analysis. M2 (mg) shows the mass of CFAMEs layer which was weighed after the washing and separation process for each isomerization experiment. The fatty acid composition of the samples before and after the isomerization was then identified by comparison of the peaks with retention time of the respective external standards.
2.2.7. Ultra-Violet (UV) Spectrophotometry Sample Preparation and Analysis
The total conjugated dienoic acid content (w/w %) of the FAMEs which was measured by GC after the isomerization was further validated using a spectrophotometer (Ultraspec 3100 pro UV/Visible: Amersham Biosciences, Biochrom Ltd., Cambridge, England) and according to the method described in American Oil Chemists’ Society Official Method (2006) with slight modification [20] . 0.02 - 0.04 g of each isomerized sample weighed into a 25 mL volumetric flask and dissolved in isooctane. The volume was made up to the mark with the same solvent, and the solution was completely mixed and then allowed to stand 15 min at 32˚C to reach the temperature equilibrium. Then, the presence of the conjugated double bonds was confirmed and the quantity of the total CLAMEs (% w/w) in each isomerized samples calculated. The absorbance of the samples measured using quartz cuvettes (1 cm × 1 cm × 4.5 cm) at 32˚C. Firstly, the presence of the conjugated dienes was verified by identification of the maximum absorbance of the samples at 233 nm region after scanning each sample from 200 nm to 350 nm. The absorbance of each sample was then measured and recorded at 233 nm. The measurements were triplicated, and average considered as the absorbance of each sample. The mass percentage of CLAMEs for each sample was calculated as follows:
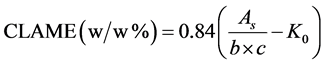 (4)
(4)
where As shows absorbency at 233 nm, b and c represent the cuvette length (cm) and the concentration of the particular sample (g/L), respectively. K0 is the absorptive constant of CLAME (i.e., 0.07).
2.2.8. Statistical Analysis and Model Fitting of the Isomerization
The experimental and predicted values of the total CLAMEs were calculated as the response at the points based on the experimental design. The experimental data were subjected to analysis of the variance (ANOVA) using the response surface regression procedure. Such analysis was carried out to determine the statistical significance of the isomerization parameters that affected the response, and to fit a regression relationship between the experimental data and independent variables for developing a model. A second-order polynomial equation was used to describe variations in response variables. As the response was the total CLAMEs (Y, % w/w), the generalized response surface model is given below:
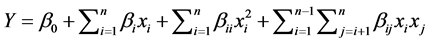 (5)
(5)
where Y is the predicted response, β0 is the offset term, βi is the linear coefficient, βii and βij are the quadratic and interaction coefficients, xi and xj are the independent variables [8] . In this study, four factors were engaged in the isomerization reaction; thus, the overall relationship between the factors and the response is
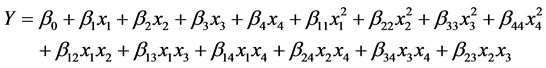 (6)
(6)
2.2.9. Optimization and Verification of the Isomerization Process
The numerical optimization process was performed using a response optimizer to get to the optimal point, which resulted in the desired response. Considering the mass percentage of the LAMEs is 72.90% (Table 2), the target response in pro- duction of the total CLAMEs is regarded to be 72.90% as well meaning that the best response be achieved when the LAMEs completely isomerizes into the CLAMEs. The adequacy of the regression model was verified by comparing the experimental data with predicted values using a two sample t-test [21] .
3. Results and Discussion
3.1. GC Analysis on FAMEs and CFAMEs Samples
The fatty acid profile of the sunflower oil obtained from GC analysis expressed
![]()
Table 2. The fatty acid content of the sunflower oil before and after the isomerization process.
aThe fatty acid composition of the transesterified sunflower oil (i.e., FAMEs) obtained from the optimum conditions of the transesterification (Koohi Kamali, Tan & Ling, 2012); bThe fatty acid composition of the of sunflower oil after the isomerization process (i.e., CFAMEs) resulting from the optimum conditions of the isomerization. Data represent mean ± SD (standard deviation of 3 analysis); cTotal conjugated linoleic methyl esters (w/w %) was calculated as sum of the CLA isomers; dNot detected.
in Table 2. A remarkable difference between the fatty acid composition of the sunflower oil before and after the isomerization is the presence of CLAMEs in the isomerized mixture (i.e., average of 70.4% under the optimum conditions). This indicates that the concentration of CLAMEs increased from 0% to 70.40% in the sample obtained from optimum isomerization condition. However, the concentration of the linoleic acid (c9, c12-Octadecadienoic acid) decreased from 72.9% to 1.50%. These results showed that the LAMEs were converted into that of conjugated isomers during the isomerization. The degree of isomerization (% w/w) or the conversion degree of the LAMEs into CLAMEs was calculated using Equation (7).
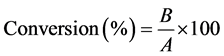 (7)
(7)
where B is the total mass percentage of the CLAMEs in isomerized sample, and A is the mass percentage of the LAMEs in the transesterified fraction which was used as the substrate of the isomerization. The degree of the isomerization was 96.6% under the optimum condition showing that 96.6% of the LAMEs were converted into CLAMEs. In addition, the content of CLA isomers was quantified using GC (i.e., 70.40%) half of which was 9-c, 11-t and 10-t, 12-c-octadecadi- enoic acid in the sample derived from the optimum conditions. Table 2, is showing the fatty acid composition of the transesterifed sunflower oil and the isomerized one under the optimum conditions. As can be observed in Table 2, the content of the linolenic acid which was 0.2 (before isomerization) is decreased to 0% in the isomerized mixture. However, the palmitic, palmitoleic, stearic and oleic contents are not significantly changed (p > 0.05).
3.2. GC Analysis on CLA before and after the Urea-Treatment
Table 3 shows the fatty acid composition of the isomerized sunflower oil (from optimum conditions) or before the purification, after the 1st and the 2nd urea- treatment crystallization.
During the 1st stage of the urea crystallization, the saturated and the monounsaturated fatty acids are trapped into urea channel shape of hexagonal crystalline
![]()
Table 3. The mass percentage of the fatty acids in isomerized mixture of the sunflower oil after the first and the second stages of the urea-inclusion crystallization.
Data are the average of 3 analysis ± SD (standard deviation). ND; represents not detected.
complexes and can be removed from the mother liquor using filtration techniques. However, due to the smaller bulk density of saturated fatty acids than that of monoenoic, the crystallization rate of saturated fatty acids with urea is faster compared with the monoenoic fatty acids. As can be observed in Table 3, although the amount of saturated fatty acids (i.e., C16:0 and C18:0) and oleic acid (C18:1) decreased after the 1st stage of purification, small amounts of these fatty acids remained in the mixture (Figure 1(a)). The amount of the oleic acid decreased drastically at the end of the second crystallization step; however, the saturated fatty acids were completely removed from the isomerized mixture at this stage. The corresponding GC peaks related to the saturated fatty acids disappeared in the second stage of the urea-treatment (Figure 1(b)). These observations supported the results from Harris (1996) and Hayes, Alstine & Asplund (2001) who indicated the strong attraction between the urea molecules and the trapped guests in the urea circular channel as UICs that can thus, be removed from the mother liquor without loss of integrity [22] [23] .
![]()
![]()
Figure 1. Gas chromatogram of the purified conjugated linoleic acid after the urea-inclusion crystallization, showing the fatty acid profile of the mixture after the first (a) and the second (b) stage of the purification.
In the 2nd stage of the urea crystallization, mixtures of unsaturated fatty acids including CLA isomers and the residual amount of saturated and mono-unsa- turated fatty acids which did not form the complex in the 1st crystallization were subjected to the urea-inclusion crystallization. The CLA isomers began to form the CLA-UIC crystals and concentrated CLA was isolated from the mixture. However, alpha-linolenic acid (LNA) remained in the mother liquor and supported the earlier findings which indicated that the poly-unsaturated fatty acids with large bulk densities cannot be locked up in crystallized urea channels and remains in the liquid mixture [16] [22] [24] [25] .
The purity of the CLA isomers, which was 80.54% after at the first crystallization step increased to 95.5% by the end of second stage of the purification (Table 3). Wherein, 45.70% and 49.80% of the CLA has been determined to be of cis-9, trans-11-CLA and trans-10, cis-12-CLA, respectively. These results are also confirmed by the observations of Kim et al. (2003), who obtained the CLA with purity of 95.5% after the second stage of the crystallization [24] . A possible interpretation for increment of the CLA purity in the 2nd crystallization might be its higher bulk density due to 2 double bonds as compared to the saturated and monoenoic fatty acids which makes it more difficult to be attached to the urea crystal channels.
3.3. UV Spectrophotometry Analysis of CLAMEs Samples
Since the conjugated double bonds reported to have a strong absorption in the region of 233 nm, formation of conjugated dienes after the alkali-isomerization was confirmed with UV spectrophotometric assay and for validating the GC results [4] . The comparison between two series of the measurements which were obtained from the spectrophotometric and GC analysis was made using t-test. Two series of the data were consistence and no significant difference was found between the values of the total CLAMEs (% w/w) resulted from GC and spectrophotometric assay (p > 0.05). From Table 2, the linolenic acid (C18:3) which was 0.2% before the isomerization diminished and was not even detectable after the course of the isomerization. Since the spectrophotometry results were close to that of the results obtained from GC analysis, it could be concluded that the total conjugated fatty acids measured were of the conjugated linoleic acid (C18:2) and not related to the conjugated linolenic acid (C18:3) methyl esters.
3.4. Statistical Results and Model Fitting
The mass percentages of the total CLAME for each treatment were calculated using Equation (2) and reported as the response of each isomerization treatment. The experimental data and the predicted values obtained from the software after fitting (i.e., reducing) the regression model are listed in Table 4. The estimated regression coefficients, corresponding R values of the fitted-reduced model, the F-ratios and the p-values of the terms with significant effects (p < 0.05) for linear, quadratic and interaction effects obtained from ANOVA and are shown in Table 5.
![]()
Table 4. The experimental and predicted responses for production of total conjugated linoleic acid methyl esters.
aNo significant differences (p > 0.05) between experimental (Y0) and predicted values (Yi); bThe predicted values are calculated by the software and are resulting from the reduced fitted model; cY0 - Yi: residual.
The terms with non-significant effects on the response (p ≥ 0.05) were dropped from the initial model and the experimental data were re-fitted with the significant terms (p < 0.05) (Table 5) [26] . According to the p-values of the fitted
![]()
Table 5. Regression coefficients, R2, F-ratio and p-value of the final reduced model.
aThe non-significant term (p > 0.05) which were further eliminated from the regression fitted model. 1) Time of the reaction (min); 2) Temperature (˚C); 3) Mass percentage NaOCH3 in total reaction mixture (% w/w); 4) Mass percentage of PEG400 in total reaction mixture (% w/w).
model terms (Table 5), the main or single effects of the reaction temperature (x2), NaOCH3 (x3) and PEG400 (x4) together with the quadratic terms of the temperature (x2 * x2) and PEG400 (x4 * x4) concentration with significant effect (p < 0.05) on the variability of response (y, % w/w) were kept in the final reduced model. However, the linear effect of the reaction time (x1) and the interaction effects of the factors were removed due to their non-significant effects on the response (p ≥ 0.05). The final fitted model indicating the production yield of the total CLAMEs as a function of the independent variables is established as follows:
 (8)
(8)
where Y is the response, x2 is the temperature, x3 and x4 are the concentrations of the NaOCH3 and the PEG400, respectively. The adequacy of the regression model was verified by an analysis of the model and through the measurement of the coefficient of determination (R2) [21] . The value of R2 for the reduced model (Equation (8)) was 0.888 showing that the final reduced model (R2 > 0.8) was adequate. From the value of R2, it can be concluded that the model is able to predict 88.8% of the variations in production the total CLAMEs [27] .
3.5. Effect of the Reaction Time (x1)
The reaction time was one of the main parameters used in many studies for chemical synthesis of CLA [28] [29] . In order to evaluate its effectiveness on variation of the response, the time was employed (90 - 330; min) (Table 1). The result of the statistical analysis on the data revealed that neither the linear nor the quadratic terms of the reaction time had significant influences on production of CLAMEs as response (p ≥ 0.05) (Table 5). Therefore, this variable excluded from the regression model and the final model was re-fitted with only the significant terms (p < 0.05) (Equation (8)) [9] . The time was kept constant at its centre point value (210 min) during the isomerization under the optimum conditions. These outcomes were in similarity with the research highlights conducted by Yang and Liu (2004), who concluded that the linear or the quadratic term of the time have no significant effect on the isomerization (p ≥ 0.05) [17] .
3.6. Effect of the Reaction Temperature (x2)
The temperature was varied (100˚C - 180˚C) to evaluate the effectiveness of this factor on response. The analysis of the variance showed that the linear and quadratic terms of the reaction temperature significantly affected the total CLAMEs (% w/w) production (p < 0.05) (Table 5). The quadratic term of the reaction temperature with the smallest coefficient of determination (−0.007), confirmed that the temperature had the lowest significant effect on response among other factors. The negative sign of the quadratic coefficient indicated that this term adversely affected the total CLA production. However, the positive sign of the estimated coefficient for the liner term of the reaction temperature (+2.206), confirmed that the main term of the temperature has positively affected the response. A positive effect means that when the reaction temperature increases from 100˚C to 140˚C, more CLA is formed. Moreover, when the temperature increases beyond its center point (>140˚C), the opposite trend is observed and the production of total CLAMEs would decrease (Figure 2). These results were
![]()
Figure 2. The response optimizer graph, showing the optimum points which lead to the best response (i.e., production of the total CLA methyl esters with target value of 72.90% based on the mass percentage of total fatty acid methyl esters mixture.
in similarity with findings by Yang and Liu (2004), where the quadratic term of the temperature has been shown to have a negative effect on production of CLA isomers [17] . Therefore, when the value of the reaction temperature is set at 140˚C, the production of total CLAMEs reaches its maximum (72.90%). This optimum value was within the temperature range of the isomerization already suggested by Wenk and Haeser (2005) [30] .
3.7. Effect of NaOCH3 (x3)
The NaOCH3 that was used as a preferred catalyst for a prototropic double bonds shifting in isomerization has been broadly applied in commercial organic syntheses by numerous researchers and has been found to be capable of catalyzing the reactions at low temperatures [6] [12] [30] [31] [32] . The single term of the mass percentage of NaOCH3 has shown to have significant effect on production of total CLAMEs (p < 0.05) (Table 5). However, the effect of the quadratic term of this variable has not significantly influenced the response (p ≥ 0.05). Regarding the magnitude of the F-ratio for the linear term of NaOCH3 (50.83) and the positive sign of the estimated regression coefficient (+4.96), it could be concluded that the linear term of NaOCH3 concentration was the secondary determining factor that positively affected the total CLAMEs production. This means that by increasing the concentration of NaOCH3 from 1% to 5%, the production of total CLAMEs enhanced (Figure 2). This result showed that the optimum value of NaOCH3 that was 5% based on the mass percentage of the total reaction mixture, was sufficient to achieve the maximum response. This value was also the maximum level of the sodium methoxide within the range which has been suggested by Wenk and Haeser (2005) [30] .
3.8. Effect of PEG400 (x4)
The PEG400 was used to improve the degree of the isomerization in a solvent free system and was chosen since it is non-toxic and commonly used in pharmaceutical and medicinal industries. In addition it can be completely removed from the mixture with strong phosphoric acid [11] . As a PTC, PEG400 accelerates the isomerization process by combining the two immiscible phases. This compound is able to encapsulate the alkali metal cations of the basic catalysts (e.g., Na+ from sodium methoxide) in its hydrophilic interior, and further attach to methyl esters through its hydrophobic exterior [33] .
According to the positive sign of the corresponding coefficients (Table 5), the linear and the quadratic terms of the PEG400 concentration had a positive significant effect on the isomerization (p < 0.05). The linear term of the PEG400 with the highest magnitude of F-ratio has been found to be the primary determining factor that affected the response. In other words, the main term of the PEG400 had the most significant effect (p < 0.05) on total CLAMEs production among other reaction parameters (the highest value of F-ratio in Table 5). As the concentration of the PEG400 was increased from 0% to its centre point (1; % w/w), the production of the total CLAMEs enhanced (Figure 2). In addition, the maximum response was observed at 1.06% of the PEG400 which was considered as its optimum value (Figure 2). When, PEG400 was used in concentrations above the optimal level which was enough to get to the maximum response, changes in the response were not remarkable (Figure 2). Therefore, it is reasonable to keep the amount of the phase transfer catalyst in its optimal level with regards to the economical considerations. The isomerization degree was measured using Equation (7). The results showed that the isomerization degree that was 48.8% in the absence of PEG400 (treatment number 15), reached 96.6% at the optimum condition when the amount of PEG400 was adjusted to its optimal level (1.06%). This finding supports the previous results of Wenk and Haeser (2005) [30] , where the degree of the isomerization was demonstrated to be improved by using a PTC. It can be interpreted that PEG400 increased the solubility of the NaOCH3 in the fatty acid esters layer, and thus accelerated the reaction between two phases. The reason for a greater degree of the isomerization as a result of the optimum isomerization conditions from this study (i.e., 96.6%) compared that of obtained in previous studies [4] [30] (i.e., 90%) was due to the optimization of the isomerization.
3.9. Optimization and Verification of the Response Surface Model
Based on the numerical optimization, the best combination of the reaction conditions (optimum point) for the maximum production of total CLAME (72.90%) was at the reaction temperature of 140˚C, 5% (w/w) NaOCH3, and 1.06% (w/w) PEG400 based on the weight of the total mixture (Figure 2). The experimental data were compared with those predicted by the software using t-test analysis. The experimental responses were shown to be close to the predicted ones (p ≥ 0.05) (Table 4). This result verified that the reduced regression model was adequate (Equation (8)). The fitted line plot of the data shows the correlation between experimental and predicted responses (Figure 3). For validation of the
![]()
Figure 3. The fitted line plot of the predicted and the experimental responses, showing the closeness of predicted (Yi) and experimental (Y0) values of the total CLAME (% w/w).
method, the sample empirically produced under the optimum conditions was then analyzed by GC and the result was compared with that of predicted by the software using t-test analysis. The value of the total CLAME (72.90%) which was predicted by the software was compared with the actual amount of the total CLAME (70.40%) that was produced under the optimum conditions (Figure 2). Based on the two-sample t-test result, no significant differences (p ≥ 0.05) between the experimental and predicted values observed. This outcome showed that the method was valid and the reduced fitted model obtained by RSM was suitable to predict the total CLAME production [21] .
4. Conclusion
In this research, optimization of the production of two beneficial isomers of CLA was investigated. Isomerization of the FAMEs of the sunflower oil was investigated. To optimize the isomerization condition, the effect of four variables namely reaction temperature, the concentration of PEG400 and NaOCH3 on production of total CLAME was studied. The reaction temperature, the concentration of PEG400 and NaOCH3 were found to have positive influence on the response. However, the effect of the concentration of PEG400 was found to be more significant on response than those of other factors. The quadratic effect of the temperature was negative on response. Despite the upper limit of the reaction temperature already suggested by Wenk and Haeser (2005) was 150˚C, in this study the reaction temperature of 140˚C was the optimum temperature which resulted in the best response [30] . Furthermore, the application of the temperatures beyond 140˚C has been found to adversely affect the production of total CLAME. The reaction time and the interactions between the factors had no significant effect on the production of the CLAME (p ≥ 0.05). The optimum point for the maximum production of CLAME (72.90%) was at a combined level of 5% w/w NaOCH3, 1.06% w/w PEG400 and the reaction temperature of 140˚C. No significant differences was observed between the experimental and the predicted responses (p ≥ 0.05) using a two sample t-test. This result verified that the response surface model which was established for predicting the response as a function of the variables was adequate. The degree of the isomerization after the process optimization was greater than those obtained from the non-optimized isomerization conditions. Therefore, by the application of the response optimizer in the numerical optimization, the initial settings of the optimal levels of the variables were sensitively modified and the levels were adjusted again to improve the process response. A second-order model with a high R2 (>0.80) was successfully developed to describe the relationships between the total CLAME as the response and the independent variables. As expected in this study, the degree of isomerization that was 90% in non-optimized methods was improved and reached 96.6% after the process optimization using RSM.
Acknowledgements
I express my deep gratitude towards the almighty god for providing this opportunity to complete this study. I thank all the personal who supported me with their knowledge and experience.