Removal and Recovery of Chromium(III) from Aqueous Chromium(III) Using Arthrobacter nicotianae Cells ()
1. Introduction
Chromium is used in the textile, leather tanning, electroplating, metal finishing, wood treatment, corrosion control, oxidation, and anodizing industries [1] . High levels of chromium absorbed by the body can generate serious health issues and a concentration of 100 μg/g body wt. can ultimately be lethal [2] . Currently, the main processes for the elimination of chromium are adsorption, reverse osmosis, and chemical reactions that involve reduction and precipitation [1] . Among these processes, adsorption is a feasible method for removing traces of chromium from wastewater [1] and many adsorbents have been examined for this purpose [2] [3] .
Adsorption is the most effective and widely used technique for the removal of toxic heavy metals from wastewater [4] . Although activated carbon has been commonly used for this purpose, it is limited by its high cost and difficult procurement [1] . Accordingly various low-cost substances, like fly ash [5] , wood charcoal [6] , bituminous coal [7] , bagasse and coconut juice [8] , rice husk carbon [9] , peat [10] , red mud [11] , used black tea leaves [12] , activated carbon from sugar industrial waste [13] and sugarcane bagasse [14] have been examined.
We previously demonstrated that microorganisms are able to remove many toxic and useful metals, such as lithium [15] , uranium [16] , thorium [17] , rare earth metals [18] , and gold [19] from aqueous solutions. Additionally, immobilized persimmon tannin gel removes gold(III) from a hydrogen tetrachloroaurate(III) solution [20] . Microorganisms could remove small amounts of chromium from a chromium(VI) solution; however, the amount of chromium removed using persimmon tannin gel is much larger than that using microbial cells [21] . Most Cr(VI) is removed using persimmon tannin gel, but some chromium remains in the form of Cr(III) in the solution.
Despite of the Cr(III) removal using persimmon tannin gel under various experimental conditions, little improvement in the removal efficiency has been observed.
Therefore, the removal of Cr(III) using microorganism, Arthrobacter nicotianae which can remove a large amount of metals [15] [16] [18] , was examined in this paper.
2. Material and Methods
2.1. Culture of Microorganisms
Microorganisms were grown in medium containing 4 g/L meat extract, 5 g/L peptone, and 5 g/L NaCl in deionized water. The cultures of microorganisms, maintained on agar slants, were grown in 300 mL of the medium in a 500 mL flask with continuous shaking (120 rpm) at 30˚C. To ensure a sufficient amount of resting microorganisms after separation from the growth medium, the cultures were grown for 72 h.
Cells were collected by centrifugation (10,000 rpm) at 20˚C for 10 min, washed thoroughly with deionized water, and used in subsequent removal experiments.
2.2. Immobilization of Microorganism
Five grams of precultured cells were suspended in 4.5 mL isotonic sodium chloride solution, and 680 mg of acrylamide monomer, 34 mg of N,N’-methy- lene-bis(acrylamide), 0.3 mL of 3-dimethylaminopropionitrile solution (5%), and 0.34 mL of potassium persulfate solution (2.5%) were added to the suspension.
After solidification, the gel was crushed into small pieces (50 - 100 mesh), washed thoroughly with isotonic sodium chloride solution followed by deionized water, and used for adsorption experiments.
2.3. Cr(III) Removal for Various pH Values, Cr(III) Concentrations, and Cell Amounts
Cr(III) nitrate was used. The pH of the solution was adjusted to the desired value (1.0 - 5.0) using 0.1 M HCl. Resting cells (15 mg dry wt. basis) were suspended in 100 mL solutions containing 100 μM (5 ppm) Cr(III) (pH 1 - 5) for 1 h at 30˚C to examine the effect of pH. Similar experiments containing 19 - 960 μM (1.0 - 50 ppm) of Cr(III) (pH 5), or resting cells (5.0 - 60 mg dry wt. basis) were also performed to examine the effect of concentration or cell amount, respectively. Microorganisms were then collected by filtration through a nitrocellulose membrane filter (pore size 0.2 μm). Control studies confirmed that the free metal was not adsorbed on the filter.
The amount of Cr(III) removed by cells was determined by measuring the difference between the initial and final metal content in the filtrate using an atomic absorption quantometer (AA-6300; Shimadzu Corporation, Kyoto, Japan).
2.4. Time Course of Cell Amount on Cr(III) Removal Using A. nicotianae Cells
Resting cells (15 mg dry wt. basis) were suspended in 100 mL solutions containing 5.0 ppm (100 μM) Cr(III) (pH 5) for 5 min to 24 h at 30˚C.
2.5. Quantitative Analysis of the Selective Removal of Seven Metal Ions Using A. nicotianae Cells
A. nicotianae cells (15 mg, dry wt. basis) were suspended in 100 mL of a solution (pH 5.0) containing 4 × 10−5 M Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Cr3+ as nitrates for 1 h at 30˚C.
2.6. Chromium Adsorption/Desorption from a Cr(III) Solution Using Immobilized A. nicotianae Cells
The extent of Cr adsorption/desorption from a Cr(III) nitrate solution using immobilized A. nicotianae cells was investigated. Immobilized A. nicotianae cells (15.4 mg dry wt. cells basis) were suspended with 100 mL of Cr(III) (4.68 ppm, pH 5.0) for 1 h at 30˚C. Immobilized A. nicotianae cells that adsorbed Cr(III) were separated from the suspended solution by filtration through a membrane filter (pore size 0.2 μm) and suspended with diluted HCl or Na2CO3 solution (0.01, 0.1, or 1 M) for 1 h at 30˚C.
2.7. Effect of Temperature on Cr(III) Desorption from a Cr(III) Adsorbed Immobilized A. nicotianae Cells
Immobilized A. nicotianae cells (15.7 mg dry wt. cells basis) were suspended with 100 mL of Cr(III) (5.19 ppm, pH 5.0) for 1 h at 30˚C. Immobilized A. nicotianae cells that adsorbed Cr(III) were separated following the same method described in Section 2.6 and suspended with diluted HCl (0.1 or 1 M) for 1 h at 30˚C-(refluxed temperature).
2.8. Recycling of Cr(III) Removal and Recovery
Cr(III) solution (5.26 ppm, pH 5.0, 50 mL) was passed through the immobilized A. nicotianae cells (230 mg dry wt. cells basis) column (diameter 8 mm) at 30˚C. Then, immobilized A. nicotianae cells that adsorbed Cr(III) were subjected to desorption by applying 0.1 M HCl at reflux temperature for 1 h using a batch system.
3. Results and Discussion
3.1. Effect of pH on Cr(III) Removal from Aqueous Cr(III) Solution Using A. nicotianae Cells
The effect of pH on Cr(III) removal from aqueous Cr(III) as Cr(NO3)3, using A. nicotianae cells was examined. As shown in Figure 1, the percentage of Cr(III) removed from the solution was maximal (approximately 90%) at pH 5. For 2.5 ppm, compared with Cr(VI) using persimmon tannin gel [21] , the amount of Cr(III) removed using A. nicotianae was six times higher.
In contrast, the zeta potential of A. nicotianae was decreased as the pH of the solution increased [22] . These results indicates that Cr(III) removed (%) depends on the charge of the surface of A. nicotianae cells and solution.
3.2. Effect of Cr(III) Concentration on Cr(III) Removal from Aqueous Cr(III) Using A. nicotianae Cells
The effect of Cr(III) concentration on Cr(III) removal was examined. As shown
![]()
Figure 1. Effect of pH on the removal of Cr(III) by A. nicotianae cells. Symbols: Squres, Cr(III) removed (%), Circles, Zeta potential of A. nicotianae cells (mV) [22] .
in Figure 2, Cr(III) removal (μmol/g dry wt. cells) increased as the Cr(III) concentration increased, while the Cr(III) removed (%) decreased. Cr(III) removed was almost quantitatively for a low Cr (III) concentration, such as 1.0 ppm (19 μM) Cr(III) solution. The amount of Cr(III) removed for a high Cr(III) concentration, such as a 960 μM (50 ppm) Cr(III) solution (equilibrium concentration, 860 µM (45 ppm)) was approximately about 720 µmol Cr(III)/g dry wt. cells.
The relationship between the residual Cr(III) concentration in the solution and the amount of Cr(III) removed is shown in Figure 2. The line for Cr(III) removed (μmol/g dry wt. cells) represents the Langmuir isotherm. It is evident from this figure that Cr(III) removal using A. nicotianae cells obeys the following Langmuir isotherm over the whole range of concentrations tested:
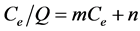 (1)
(1)
where Q indicates the amount of Cr(III) removed (μmol Cr(III)/g dry wt. cells), Ce is the residual Cr(III) in the solution (μM Cr(III)), and m and n are Langmuir constants. It was estimated that Ce/Q = 1.38 × 10−3 Ce + 2.25 × 10−2. The maximum amount of Cr(III) removed (μmol Cr(III)/g dry wt. cells) estimated from the slope of the line was 724 μmol Cr(III)/g dry wt. cells.
3.3. Effect of Cell Amounts on Cr(III) Removal from Aqueous Cr(III) Using A. nicotianae Cells
The effect of cell amounts on Cr(III) removal from aqueous Cr(III) solution using A. nicotianae cells was examined. As shown in Figure 3, the percentage of Cr(III) removed increased as the cell amounts increased whereas the Cr(III) removed (μmol/g dry wt. cells) decreased. The Cr(III) removed from the 200 μM (10 ppm) solution using over 40 mg (dry wt. basis) of A. nicotianae cells was
![]()
Figure 2. Effect of Cr(III) concentration on the removal of Cr(III) by A. nicotianae cells. Symbols: closed squares, Cr(III) removed (μmol/g dry wt cells); open squares, Cr(III) removed (%).
![]()
Figure 3. Effect of cell amounts on the removal of Cr(III) by A. nicotianae cells. Symbols: closed squares, Cr(III) removed (%), opend squares, Cr(III) removed (μmol/g dry wt cells).
quantitatively reduced, and the amount of Cr(III) (μmol/g dry wt. cells) using less than 20 mg of the cells was approximately 600 μmol Cr(III)/g dry wt. cells.
3.4. Time Course of Cr(III) Removal from Aqueous Cr(III) Using A. nicotianae Cells
The removal of Cr(III) using A. nicotianae cells was examined in a time course analysis. These results are summarized in Figure 4. The amount of Cr(III) removed (%) using A. nicotianae cells increased very rapidly, and 76% of the Cr(III) in the solution was removed during the first 5 min following the supply of Cr(III). The removal of Cr(III) reached an equilibrium within 6 h.
3.5. Selective Removal of Cr(III) Using A. nicotianae Cells
To determine which heavy metal ion can be most readily removed using A. nicotianae cells at pH 5, the selective removal of heavy metal ions from a solution containing 4 × 10−5 M Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Cr3+ was examined. As shown in Figure 5, the relative degree of heavy metal ion adsorption by A. nicotianae cells was Cr3+ = Cu2+ >> others, indicating that A. nicotianae cells can remove equal amounts of Cr(III) and Cu(II) more readily than other heavy metal ions.
3.6. Adsorption and Desorption of Cr(III) from Cr(III) Solutions Using A. nicotianae Cells
The amount of Cr(III) removed using A. nicotianae cells was strongly affected by the pH of the solution. The amount of Cr(III) removed (%) increased as pH of the solution increased. Therefore, the adsorption of Cr(III) was examined at pH 5 and desorption was examined using both acidic and alkaline conditions by a batch system. As shown in Table 1, most of the Cr(III) (333 - 343 μg, 71.2% -
![]()
Figure 4. Time course of the removal of Cr(III) by A. nicotianae cells.
![]()
Figure 5. Selective removal of heavy metals by A. nicotianae cells.
![]()
Table 1. Desorption of Cr(III) from A. nicotianae cells after Cr(III) adsorption using diluted HCl or Na2CO3.
73.3%) was adsorbed on A. nicotianae cells. However, only a small amount of adsorbed Cr(III) was desorbed in acidic conditions (i.e., 35% using 0.01 M- and 45% using 1 M HCl at 30˚C) and alkaline conditions (i.e., 4% using 0.01 M- and 24% using 1 M Na2CO3 at 30˚C).
3.7. Effect of Temperature on the Desorption of Adsorbed Cr(III) Using Immobilized A. nicotianae Cell
The amount of Cr(III) desorbed was very low using A. nicotianae cells at 30˚C. Therefore, the effect of temperature on the desorption of adsorbed Cr(III) was examined. As shown in Figure 6, Cr(III) desorbed (%) increased as the temperature increased above 60˚C. Most (99.1%) of the adsorbed Cr(III) was desorbed at reflux temperature.
3.8. Cycling of Cr(III) Removal and Recovery
To obtain basic information on the recovery of Cr(III) using immobilized A. nicotianae cells, cycles of Cr(III) adsorption and desorption was repeated five times.
As shown in Figure 7, Cr(III) was quantitatively adsorbed on the immobilized A. nicotianae cells using a column system and approximately 90% of adsorbed Cr(III) was desorbed with a 0.1 M HCl solution at reflux temperature by a batch system. Most of the Cr(III) adsorbed was desorbed after 5 repetitions of the adsorption-desorption cycle in a column (adsorption)-batch (desorption) system.
![]()
Figure 6. Effect of temperature on the desorption of Cr(III) adsorbed using A. nicotianae cells by a diluted HCl solution. Symbols: Squres, 0.1 M HCl, Circles, 1 M HCl.
![]()
Figure 7. Repetition of Cr(III) adsorption-desorption cycles using immobilized A. nicotianae cells. Symbols: Squres, Cr(III) adsorbed (%), Circles, Cr(III) desorbed (%).
4. Conclusions
The removal of Cr(III) using A. nicotianae cells was strongly affected by the pH of the solution. The amount of Cr(III) removed increased as the pH of the solution increased. The maximum Cr(III) removed (%) of appropriately 90% was observed at pH 5. As the removal of 2.5 ppm Cr(III) using persimmon tannin gel was about 30% (21), the amount of Cr(III) removed using A. nicotianae was six times higher than that using persimmon tannin gel.
The Cr(III) removed (µmol/g dry wt. cells) increased as the Cr(III) concentration increased, whereas the Cr(III) removed (%) decreased.
The removal of Cr(III) using A. nicotianae cells obeyed the Langmuir isotherm over all concentrations examined.
Cr removed (%) increased as the cell amount increased, whereas the Cr(III) removed (μmol/g dry wt. cells) decreased. The amount of Cr(III) removed using A. nicotianae cells increased very rapidly and 76% of the Cr(III) in the solution was removed during the first 5 min following the supply of Cr(III). The removal of Cr(III) reached an equilibrium within 6 h. The selective removal of 7 kinds of metal ions was examined using A. nicotianae cells; Cr(III) and Cu(II) were removed at much higher rates than the other metal ions. Immobilized A. nicotianae cells can also adsorb Cr(III) at 30˚C and can desorb most Cr(III) with 0.1 M hydrochloric acid at reflux temperature. Repetition of adsorption (column) and desorption (batch) cycles using immobilized A. nicotianae cells can be repeated 5 times.
We observed substantial toxic Cr(VI) removal using persimmon gel; however, some (~20%) Cr(III) was produced, and only a low amount of Cr(III) removed [21] . Based on the results of this paper and recent results, the removals of Cr(VI and III) from the Cr(VI) wastewater system and Cr(III) products are possible using persimmon gel and A. nicotianae, respectively.