1. Introduction
Paper mill sludge is a by-product of waste water treatment process in paper mill. Rapid economic development has brought rapidly growing of paper mill sludge yield. Compared to municipal sewage sludge, paper mill sludge contains more organic substance. Therefore, disposal of paper mill sludge through incineration has been encouraged. The water content of paper mill sludge is as high as 70% - 80%, which is harmful to incineration [1] [2]. Thermal drying to reduce water content has been proved to be an effective method for reducing the volume and weight, increasing the heating value [3]. More and more attentions have been paid into the incineration of paper mill sludge in recently years, but there is little attention on the drying kinetics of paper mill sludge [4] [5] [6]. Research on drying kinetics of other materials usually adopted drying model method [7] [8], so thermal analysis method was used for discuss the drying kinetics of paper mill sludge in this paper.
Two paper mill sludge samples were selected, and the drying experiments were completed with a constant air speed of 0.96 m/s and a temperature range of 50˚C - 80˚C on a laboratory convective dryer. The drying characteristics were discussed and the drying kinetic equation was solved. In addition, the reaction rate constant of moisture transfer and the activation energy for moisture diffusion during the thermal drying process were calculated.
2. Experiments and Methods
2.1. Sample and Experiments
The sludge samples used for experiments were collected from two different workshop of a paper mill in Wuhan. Received sludge was a kind of brown and loose material with high water content (wet basis), sample A was 74.86% and sample B was 75.9%.
The experimental set up shown in Figure 1 mainly consisted of a blower, an electric heater and a drying chamber. Air was sent into electric heater by a blower whose rotational speed was controlled by a frequency converter. As a result, the air velocity in the drying chamber related to air flow was adjustable. The relationship between blower frequency and air velocity can be built by a standard anemometer before experiments. Air was heated in the electric heater and a temperature controller was used for controlling and maintaining the required air temperature of the drying chamber inlet. The accuracy of temperature controller was 1˚C. Paper mill sludge samples were spread on a salver which was placed on the electronic scale through a steadier. The electronic scale can achieve real time measuring of the sludge weight and send the data to computer with an accuracy of ±1 g and measuring range of 5 kg.
As a heterogeneous material with complex components and interior structure, little sludge sample can’t reflect the whole characteristics well, so abundant sludge of 3.5 kg was selected for experiment every time. First, drying temperature (the air temperature of drying chamber inlet) and air velocity were set by temperature controller and frequency converter, respectively. Sludge sample was made into long strip with a diameter of 8mm by special machine, and 3.5 kg sample was put on salver. In addition, little sample was selected to measure the initial water content in the thermostatic drying chamber. Sludge weight variation was recorded by electric scale at 0.5 min interval during the drying process in order to determine the drying curve. The drying data from different drying experiments were expressed as water content versus drying time and drying rate versus water content.
![]()
Figure 1. The schematic diagram of experimental equipment.
2.2. Drying Kinetics Analysis Method
The drying process of paper mill sludge can be considered as a pyrolysis reaction, water and dry substance were the two pyrolysis products. Therefore, drying experiments can be considered as a thermal gravimetric experiment at steady low temperature. Then the thermal analysis method can be used to analyze the drying kinetics.
The relationship between reaction rate and sample weight loss rate can be described by reaction kineticmechanism equation as Equation (1).
 (1)
(1)
where 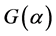 was the reaction kineticmechanism equation,
was the reaction kineticmechanism equation,  was sample weight loss rate, t was time, k was the reaction rate constant. The Arrhenius equation was used to describe the relationship of temperature and reaction rate constant, which was shown in Equation (2).
was sample weight loss rate, t was time, k was the reaction rate constant. The Arrhenius equation was used to describe the relationship of temperature and reaction rate constant, which was shown in Equation (2).
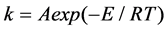 (2)
(2)
where A was preexponential factor, E was activation energy, T was thermodynamic temperature.
3. Results and Discussion
The paper mill sludge sample were dried using hot air of a temperature range of 50˚C - 80˚C and constant air velocity of 0.96 m/s, and the drying characteristics were discussed.
3.1. Drying Characteristics Curves
The variations of water content versus drying time of paper mill sludge at various drying temperature were given in Figure 2 and Figure 3. As expected, the drying temperature contributed positively to the drying rate and negatively to the total drying time. It was caused by the increase of the heat transfer and mass transfer between the air and sludge sample along with the increase of temperature.
For example, the maximum drying rate of sample A at 50˚C was 15 g/ (m2∙min) while it was 31 g/(m2∙min) at 80˚C. But the drying rate was not proportional to drying temperature, the rising speed became more and more slowly. While the drying temperature decreased from 80˚C to 50˚C, the drying time of sample A between initial and 5% water content was 260 min, 295 min, 385 min and 505 min, respectively. The drying curve can be divided to two parts, the accelerating and decreasing drying period, which was consistent with the results given by Celma [8]. The critical point between the two parts was the maximum drying rate during the drying process. The water content corresponding to the maximum drying rate dropped with the rising of drying temperature.
Results showed that the influence of drying temperature on drying rate became more and more obviously with the decreasing of water content. For example, when sludge sample A was dried at 80˚C and 50˚C, the drying rate
![]()
![]()
Figure 2. Drying characteristics at different drying temperature of sludge sample A.
was 23.8 g/(m2∙min) and 12.3 g/(m2∙min) when water content was 60%, respectively. But the drying rate was 6.5 g/(m2∙min) and 2.4 g/(m2∙min) when water content was 20%, respectively. At the earlier period of drying process, water evaporation happened on the sample surface, and interior water diffused to surface drives by humidity difference, so humidity of drying air had crucial effects on drying rate. At the later period of drying process, the sludge surface was dry, and water evaporation happened in the sludge interior. In
![]()
![]()
Figure 3. Drying characteristics at different drying temperature of sludge sample.
this case, drying temperature played the leading role in this period.
There were difference of drying characteristics between sample A and sample B. The drying time of sample A was longer than sample B, and the difference was influenced by the drying temperature.
3.2. Drying Kinetic Analysis
Drying experiments of paper mill sludge can be considered as a thermal gravimetric experiment at steady low temperature. According to the weight variation during the drying process, the sample weight loss rate was determined by Equation (3).
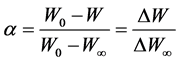 (3)
(3)
whereW0 and W were the initial weight of sludge sample and the real time weight at time t, respectively. 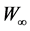 was the final weight which can be considered as the drying substance weight.
was the final weight which can be considered as the drying substance weight.  was the weight loss and
was the weight loss and 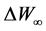 was the maximum weight loss which was the water weight in sample.
was the maximum weight loss which was the water weight in sample.
The lnln method can be used for solve the reaction kinetic mechanism equation of paper mill sludge drying process. The criterion was the line slope on Avrami-Erofeev figure. According to the Avrami-Erofeev equation , the natural logarithm equation can be given by Equation (4).
, the natural logarithm equation can be given by Equation (4).
 (4)
(4)
Then the fitting curve of  versus
can be built, and the slope of fitting curve was m. It was found that m was between 1.17 and 1.34 of sample A, while was between 1.27 and 1.38 of sample B.
versus
can be built, and the slope of fitting curve was m. It was found that m was between 1.17 and 1.34 of sample A, while was between 1.27 and 1.38 of sample B.
Compared to the common reaction mechanism equation, the suitable 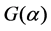 can be described by Equation (5).
can be described by Equation (5).
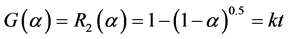 (5)
(5)
The ![]() value can be calculated by Equation (5) with the weight loss rate during the drying process at different drying temperature of two samples. The fitting curve of
value can be calculated by Equation (5) with the weight loss rate during the drying process at different drying temperature of two samples. The fitting curve of ![]() versus time t can be built, and the slope of fitting curve was reaction rate constant k. The fitting results of reaction rate constant k and coefficient of determination R2 were shown in Table 1.
versus time t can be built, and the slope of fitting curve was reaction rate constant k. The fitting results of reaction rate constant k and coefficient of determination R2 were shown in Table 1.
It was found from Table 1 that the value of reaction rate constant k varied in the range of 2.01856 × 10−3 to 4.01873 × 10−3 at temperature range of 50˚C - 80˚C of sample A, while it varied in the range of 2.41998 × 10−3 to 3.86391 × 10−3 of sample B. The values of k rose along with the rising of drying temperature.
The Arrhenius Equation (2) can be written in logarithmic form:
![]() (6)
(6)
In order to obtain the influence of drying temperature on the reaction rate
![]()
Table 1. Reaction rate constant of two sample.
constant, values of ln(k) were plotted versus 1/T shown in Figure 4. The plot was found to be essentially a straight line with a slope of −E/R.
It was found that the values of activation energy E for moisture diffusion during the thermal drying process of sample A and B were 21.53 kJ/mol and 15.38 kJ/mol, respectively, while preexponential factor of Arrhenius equation was 6.24 and 0.73. Results were similar to the activation energy of some materials in literatures given in Table 2. The activation energy of sample B was the smallest. But activation energy of sample A was higher than that of sludge of olive oil extraction but lower than that of olive pomace waste, lignite and carrot.
4. Conclusion
Drying experiments of two paper mill sludge were carried using hot air of a temperature range of 50˚C - 80˚C and an air velocity of 0.96 m/s at a laboratory convective dryer. The drying temperature contributed negatively to the total drying time, and the influence of drying temperature on drying rate became more and more obviously with the decreasing of water content. Results show the reaction rate constant k varied in the range of 2.01856 × 10−3 to 4.01873 × 10−3 at temperature range of 50˚C - 80˚C of sample A, while it varied in the range of 2.41998 × 10−3 to 3.86391 × 10−3 of sample B. The dependence of the
![]()
Figure 4. Influence of drying temperature on the reaction rate constant.
![]()
Table 2. Activation energy of some materials.
reaction rate constant on the drying temperature was given by Arrhenius equation, and the activation energies for moisture diffusion in paper mill sludge sample A and B were found to be 21.53 kJ/mol and 15.38 kJ/mol, respectively.
Acknowledgements
This work was supported by Natural Science Foundation of Hubei province (2015CFB366), Science Research and Development Project of Yichang (A15- 302-a09), the Key Laboratory of Renewable Energy Electric-Technology of Hunan Province (2015ZNDL002).