Geochemical Color Maps of the Dhaka Water, Bangladesh—New Map Presentations for Toxic Metals and Isotopes ()
1. Introduction
(http://www.dhakatribune.com/bangladesh/2016) will critically stress already limited water supplies by DWASA [1] .
The alternate future water source is surface water. In Dhaka case, urbanization concentrates domestic pollution and it is known that toxic heavy metals in surface and groundwater are directly correlated to heavy metals loading fluxes from direct discharge of wastewater from industries and polluted rain sources [2] [3] . Hazaribagh is home to 95% of Bangladesh’s leather tanneries, which every day dump 22,000 cubic liters of toxic waste into Buriganga River, the city’s main river and water supply [4] .
Surface and groundwater quality will become the principal limiting factor for sustainable development in many countries early in this century [17] . Over-ex- ploitation of the aquifer has led to a progressive decline in Dhaka Water levels. The resulting cone of depression is thought likely to be causing the infiltration of polluted surface water. Stable isotopic techniques can be used to characterize the hydrogeology and water sources the aquifer beneath Dhaka. Environmental isotope (δ18O versus δ2H) distributions can approaches identify the polluted River Buriganga as the main threat to groundwater quality, indicating priorities for monitoring and aquifer protection.
This is the first time; we determined the presence of uranium and cesium in rain, river and groundwater in Dhaka Urban by using ICP-MS analyzer. U238 is the most common isotope of uranium found in nature and the head of the uranium decay series and the 238U final stage had been replaced with lead [18] . Uranium isotopes (U234, U235, U238) are radioactive and poses health hazard. In contrast, uranium-238 cannot sustain a chain reaction, but it can be converted to plutonium-239.
This paper delineated the geochemical patterns for both major and trace elements in Dhaka Water. We have, therefore, drawn new maps for major ions, δ18O versus δ2H, toxic heavy metals and radioactive isotopes using computerized software techniques as Generic Mapping Tools (GMT), Ocean Data View (ODV) and find the current geochemical pattern. This type of map may be used to establish general baselines against which more specific natural geochemical variations and human-induced perturbations can be appraised.
2. Study Area
There were total 26 sample sites were subdivided into two groups to reflect contrast between ground and surface water chemistry (Figure 1(a) and Figure 1(b)). Stations 1 - 18, were located in central Dhaka, in Buriganga River and in river bank sites (90.300/90.500E, 23.70/23.90N) were occupied on 1st November 2015 (Figure 1(a) and Figure 1(b)). Dhaka is surrounded by deep, pollutant- rich river waters (Buriganga, Turag, Balu and Shitalakhya River). Water samples were collected for the analysis of dissolved oxygen (DO), heavy and radioactive metals, major ions and water tracer (δD and Oδ18). From the total samples list, seven were from discharge Drain (1, 2, 5) including Buriganga River water (6, 8, 10, 12), [1: Barui Khali, Rayer Bazar, Hazaribagh, 3: Boshila, West Dhanmondi; 5: Metro Housing, West Dhanmondi; 6: Zauchar, Kamrangirchar; 8: Bou Bazar, Godaraghat, Shahid Nagar; 10: Babu Bazar Boat Terminal; 12: Shadarghat Boat Terminal]; Groundwaters have been collected from the deep and shallow gro-
![]()
![]() (a) (b)
(a) (b) ![]() (c)
(c)
Figure 1.(a) Schematic diagram of study areas, rain, river, young ground, shallow ground and deep groundwater in Dhaka, Bangladesh. Discharge Drain (1, 2, 5) and Buriganga Riverwater (6, 8, 10, 12) [1: BaruiKhali, Rayer Bazar, Hazaribagh, 3: Boshila, West Dhanmondi; 5: Metro Housing, West Dhanmondi; 6: Zauchar, Kamrangirchar; 8: Bou Bazar, Godaraghat, Shahid Nagar; 10: Babu Bazar Boat Terminal; 12: Shadarghat Boat Terminal]; groundwater: five deep groundwater (2, 4, 9. 11, 13), [2: BaruiKhali, Rayer Bazar, Hazaribagh; 4: Boshila; West Dhanmondi; 9: Bou Bazar, Godaraghat, Shahid Nagar; 11: Babu Bazar Boat Terminal; 13: Shadarghat Boat Terminal], one young groundwater [7: Zauchar, Kamrangirchar] and one shallow groundwater [16: Baribadh, Matikata, Dhaka] were adjacent to Buriganga River. Rainwater (14, 15, 17, 18) [14: Dhaka University; 15: Mirpur, Dhaka; 17: Baribadh, Matikata, Dhaka; 18: Railgate, Khilgaon, Dhaka]. Dhaka supply groundwater: Dhanmondi: 19, Tejgaon: 20, Mirpur: 21, Hazaribagh: 22, Banani: 23, Armanitola: 24, Motijheel: 25, Khilgaon: 26 (central Khilgaon, Balurpar, Gourlane, Nasirabad); (b) Sampling points of Buriganga River and groundwaters were close to the river bank. Sampling point 5 (discharge point): All un- treated wastes of leather industries are flushed into the Buriganga River through the drain located in point 5; (c) Diagram of water recharge and water contamination process in Dhaka Urban.
und water adjacent to the Buriganga River bank to investigate the river recharge as showing in Figure 1(a); five were collected from deep groundwater (2, 4, 9. 11, 13), [2: BaruiKhali, Rayer Bazar, Hazaribagh; 4: Boshila; West Dhanmondi; 9: Bou Bazar, Godaraghat, Shahid Nagar; 11: Babu Bazar Boat Terminal; 13: Shadarghat Boat Terminal], one was young groundwater [7: Zauchar, Kamrangirchar] and another one was from shallow groundwater [16: Baribadh, Matikata, Dhaka]. Rainwater (14, 15, 17, 18) were taken from high traffic, industrial and from new building construction areas [14: Dhaka University; 15: Mirpur, Dhaka; 17: Baribadh, Matikata, Dhaka; 18: Railgate, Khilgaon, Dhaka]; Dhaka supply groundwaters were taken from Dhanmondi: 19, Tejgaon: 20, Mirpur: 21, Hazaribagh: 22, Banani: 23, Armanitola: 24, Motijheel: 25 and Khilgaon: 26 (central Khilgaon, Balurpar, Gourlane, Nasirabad) areas. To clarify the water bodies, several symbols (R: river water, G: groundwater, Ra: rainwater) have been used with station numbers (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18) in some data set (e.g., for rainwater: Ra14, Ra15, Ra17, Ra18, Buriganga River water: R1, R3, R5, R6, R8, R10, R12, and groundwater: G2, G4, G7, G9. G11, G13, G16 etc.). Additionally, the Figure 1(b) also shows the main discharge point (R5) of industrial wastewater and the flow path to Buriganga River areas.
We also investigated the quality and hydrology of supply groundwater from seven central city of Dhaka district (Dhanmondi: 19, Tejgaon: 20, Mirpur: 21, Hazaribagh: 22, Banani: 23, Armania: 24, Motijheel: 25 and Khilgaon: 26 (water zone: center of Khilgaon, Balurpar, Gourlane, Nasirabad). Each sampling point is adjacent to site-specific pollution sources, dominated by industrialization (old Dhaka: Hazaribagh, new Dhaka: Tejgaon), new building construction (Matikata), high traffic (Motijheel, Mirpur, Khilgaon) and commercialization (Motijheel) (Figure 1(c)) in Dhaka Urban. Moreover, we also added some supply tap water sources for geochemical comparison, e.g. shallow groundwater (Saturia: far from central Dhaka), deep groundwater (Agamashi lane: old Dhaka) and groundwater (Mohammadpur).
3. Experimental
3.1. Reagents and Materials
All reagents used in this work were of analytical grade. Ultrahigh-purity nitric acid (El grade and Tamapure AA-100), and Wako Milli-Q2 (Tamapure AA-100, Ultrapur, Kanto Chemical) were used for all operations.
Reference water samples
The precision of the ICP-MS procedure and the accuracy for each run for each samples were evaluated using a spiked river water reference material (JSAC- 0302-3C) obtained from The Japan Society for Analytical Chemistry (JSAC).
3.2. Major Ions Determination
Major ions (Cations: Na, K, Mg, Ca and anions: Cl, NO3 and SO4) were determined by DIONEX1 (DX-120)-compact ion chromatography. The Charge-Bal- ance Error (CBE) values was within limit (<±5% - 10%). Alkalinity was measured on filtered groundwater samples.
3.3. Trace Metals Detection by Using ICP-MS (Agilent 7700)
Inductively Coupled Plasma Mass Spectrometer (ICP-MS, Agilent 7700. Earth Consultant Co. Ltd., Japan) procedures were performed using a 1M HNO3 (TAMAPURE AA-100) eluent containing extremely low levels of trace metals. ICP-MS standard solutions of selected elements were purchased from USA (SPEX Certi Prep, 1000 mg L-1, Cr, As, Se, B, Cd, Pb, Cu, Zn, U, Cs, Fe, Mn etc.) [16] . 1000 mg/L solution of Be, Y, Rh, Ga, In and Bi (WAKO Pure Chemical Industries Ltd.) was used as an internal standard for normalization of individual isotopic intensities (cps) to correct for variations in instrument sensitivity over the course of the experiment, and all calculations in this study employed the corrected values. The machine was equipped with nickel skimmer cones (Spectron), but the Ni background associated with the cones was not significant relative to the Ni signal of the samples and employ a collision/reaction cell (CRC) to reduce the interferences of sample matrix. The low-resolution MS mode was used for isotope measurements. Samples were pumped into the instrument at 50 µL・min−1.
3.4. Water Tracer (δD and δ18O) Measurement
The hydrogen and oxygen isotope ratios of all samples were measured using a mass spectrometer (Micromass model PRISM) housed at the University of Toyama, Japan [19] . Samples were prepared using the H2O-H2 equilibrium method with a hydrophobic platinum catalyst for δD and the H2O-CO2 equilibrium method for δ18O. Isotope ratios are expressed as δ-values, which are represented by the following Equation (Eq.1), in ‰ unit relative to V-SMOW
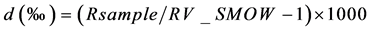
R: D/H or 18O/16O Eq.1
The obtained accuracy is better than ±1.5‰ for δD and ±0.1‰ for δ18O.
4. Results and Discussions
Dhaka Rivers are primarily fed by local rainfall, wastewater and storm water along its course through many point sources such as sluice gates, city drains and effluent outfall of the Pagla Sewage Treatment Plant (PSTP) and also receive runoff from the considerably larger Ganges-Brahmaputra-Rivers (GBR) system. All groundwater is obtained locally from underlying fluviodeltaic sands of the highly productive DupiTila Aquifer, which is locally reported to be about 140 m thick. Intensive groundwater mining from the upper DupiTila Aquifer has significantly influenced the groundwater flow direction [20] [21] . Monitoring of the spatial and temporal distribution of groundwater levels (GWL) in Dhaka City has shown a significant decrease in GWL in the central and western regions since 1980 [20] . Regional groundwater flow was directed towards the south and southeast of the city.
4.1. Geochemical and Graphical Plot for Major Ions (Piper and Stiff Diagram)
4.1.1. Stiff Diagram of Major Ions in Water Samples
Stiff Diagram is useful in making a rapid visual comparison between water from different sources [22] . It displays the concentrations of three pairs of major anion-cation constituents in rain (Ra: 14, 15, 17 and 18), river (1, 3, 5, 6, 8, 10, 12), shallow-ground (station G16, Saturia), young groundwater (station G7, Mirpur, Tejgoan, Banani) deep-groundwater (G: 2, 4, 9, 11, 13, Dhanmondi, Hazaribagh) and tap water (24: Armanitola, 25: Motijheel, 26: (Khilgaon, Gourlane, Balurpar, Nasirabad), Agamashi), plotted in units of mEqL-1 (Figure 2). Figure 2 helps to visualize the ionic composition of a water body changes (rain < river < shallow groundwater < young groundwater < deep groundwater) over space and/or time. The horizontal bar lengths represent ionic concentrations but the vertical separations of the bars and the line segments that link the bars have no physical meaning. The size of the diagram reflects the total ionic concentration. Figure 2 shows the Stiff Diagram for the Dhaka Water samples [22] [23] . The concentration of the major ions in the water samples were converted from the measured units of mg・L−1 into molar concentrations accounting for ionic charge. The Stiff Diagram shows not only the range of major ion compositions for the Dhaka Water samples, but also shows the difference of minerals in rain, river, shallow ground, deep ground and tap water types, sampled from stations 1 to 26. All diagrams are consistent with the Ca-HCO3 and Na-Ca-HCO3 water type. Deep groundwater samples from Hazaribagh (stations 2, 4, 7, 9, 11, 13) and Old Dhaka (Agamashi Lane) areas, exhibited higher sodium and calcium concentrations (20 - 85 mgL−1) than the other stations Motijheel, Dhanmondi (11 - 50 mg/L). Na-Ca-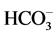 (stations 2, 4, 7, 9, 11, 13, Agamashi, Hazaribagh) water may form during silicate hydrolysis when longer residence times result in the dissolution of Na-feldspar or from the exchange of Ca for Na during evolution of groundwater along a flow path [8] . Na-Ca-HCO3-type water contains higher SiO2 and Na concentrations (Figure 2 and Table 1). Dissolved K+ appears in groundwater as a result of silicate weathering (Table 1). River water from Buriganga, such as station 1, 3, 5, 6, 8, 10 and 12, contain higher Na (154.1 - 440.2 mg/L) but low SiO2 values, indicating different source for Na+ and Cl− ions. Elevated EC (375.5 mg/L) and chloride (39.97 mg/L) in main discharge point 5 and station 8 are the marker of wastewater, provided an indication of impacts in the Buriganga Water (Figure 2 and Table 1). The water from G16 and Saturia (shallow depth wells) had narrower shaped diagrams than G9, G11 or G13 (deep ground) water, indicating low ionic concentrations due to short contact time of the water with the bedrock [8] . The Stiff Diagram for young groundwater (G7) shows a Ca-
(stations 2, 4, 7, 9, 11, 13, Agamashi, Hazaribagh) water may form during silicate hydrolysis when longer residence times result in the dissolution of Na-feldspar or from the exchange of Ca for Na during evolution of groundwater along a flow path [8] . Na-Ca-HCO3-type water contains higher SiO2 and Na concentrations (Figure 2 and Table 1). Dissolved K+ appears in groundwater as a result of silicate weathering (Table 1). River water from Buriganga, such as station 1, 3, 5, 6, 8, 10 and 12, contain higher Na (154.1 - 440.2 mg/L) but low SiO2 values, indicating different source for Na+ and Cl− ions. Elevated EC (375.5 mg/L) and chloride (39.97 mg/L) in main discharge point 5 and station 8 are the marker of wastewater, provided an indication of impacts in the Buriganga Water (Figure 2 and Table 1). The water from G16 and Saturia (shallow depth wells) had narrower shaped diagrams than G9, G11 or G13 (deep ground) water, indicating low ionic concentrations due to short contact time of the water with the bedrock [8] . The Stiff Diagram for young groundwater (G7) shows a Ca-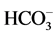 water type with a higher Ca++ and lower Na+ composition. Rainwater contained low minerals and the EC are 18 - 50 µS/cm2, however the anion concentration (
water type with a higher Ca++ and lower Na+ composition. Rainwater contained low minerals and the EC are 18 - 50 µS/cm2, however the anion concentration (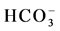 ,
,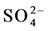 and
and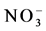 ) were little higher (5 - 10
) were little higher (5 - 10
![]()
Figure 2. Graphical presentation and classification for assessment of water quality by using Stiff Diagram. Legend; R: river water, G: groundwater, Ra: rainwater. Rainwater (Ra14, Ra15, Ra17, Ra18), Buriganga River water: R6, R8, R10, R12, Discharge point (R5, R3, R1), Groundwater: Six deep groundwater, G2 (140 m), G4 (250 m), G9 (250 m), G11 (250 m), G13; G16; one young groundwater G7, adjacent to Buriganga River; Dhaka supply groundwater: Dhanmondi: G19 (42 m), Tejgaon: G20 (67 m), Mirpur: G21 (57 m), Hazaribagh: G22 (69 m), Banani: G23 (63 m), Saturia: shallow groundwater (10 m). Tap water Armanitola: 24, Motijheel: 25, Khilgaon: 26 (Khilgaon center, Balurpar, Gourlane, Nasirabad). Agamashi.
![]()
Table 1. Physical and chemical property of rain, river and groundwater in Dhaka Urban (mg/L). Legend; R: river water (R1, R3, R5, R6, R8, R10 and R12), G: groundwater (G2, G4, G7, G9, G11, G13 and G16) and Ra: rainwater (Ra14, Ra15, G16, Ra17 and Ra18).
mg/L) in high traffic density roads (Mirpur) and commercial areas.
4.1.2. Piper Diagram for Rock Determination in Water Samples
A Piper plot is a way of visualizing the chemistry of a rock, soil, or water sample. Piper Diagrams graphically represent some of the multiple variables associated with major cation and anion data and aid in rapid determinations of rock types as well as similarities and differences in water samples [24] . The results of the process is then plotted as a trilinear diagram, as shown in Figure 3(a), where each axis varies from 0% - 100% in abundance. Figure 3(a) also shows the anion (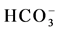 ,
,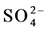 and Cl−) and cation (Na+, K+, Ca2+ and Mg2+) facies in the form of major-ion percentages and the majority of the 26 water samples. The diagram for the cation facies in Figure 3(a) shows that the majorities of the samples are dominated by Ca and are thus Ca-type water. Figure 3(b) shows the variation of ionic composition of a set of water samples for different water bodies (rain, river and groundwater). The diagram for the anion facies in Figure 3(a) shows the anion predominance in the samples and indicates that the majority (98%) of the samples lie in the range of HCO3 > 50% and can thus be considered HCO3-type water. The sequences Ca2+ > Mg2+ > Na+ and
and Cl−) and cation (Na+, K+, Ca2+ and Mg2+) facies in the form of major-ion percentages and the majority of the 26 water samples. The diagram for the cation facies in Figure 3(a) shows that the majorities of the samples are dominated by Ca and are thus Ca-type water. Figure 3(b) shows the variation of ionic composition of a set of water samples for different water bodies (rain, river and groundwater). The diagram for the anion facies in Figure 3(a) shows the anion predominance in the samples and indicates that the majority (98%) of the samples lie in the range of HCO3 > 50% and can thus be considered HCO3-type water. The sequences Ca2+ > Mg2+ > Na+ and 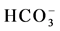 >
>  > Cl− are characteristic of relatively freshwater with a short residence time, which is indicative of shallow groundwater as reported previously for the present study area [8] . Ca− Mg− type water predominated in the samples based on our analysis. Ninety percent (90%) of the samples were categorized as Ca-Mg type. There are six main water supply zones in Dhaka City (Figure 3(c)); Zones 1 (leather industrial areas), Zones 1 and 2 are in the older more densely populated southern part of the city, Zone 4, 5 are the high traffic area, Zone 6 coincides with the major industrial district of Tejgaon located north of the old city center. Zones 1, 2 and 6 have seen the most intensive well development, but during the 1990s new drilling has increasingly extended to the other supply zones. Abundances of major ions and low dissolved oxygen (DO) 0.3 - 3.0 mg/L (Table 1) were found in Buriganga River water adjacent to industrial areas.
> Cl− are characteristic of relatively freshwater with a short residence time, which is indicative of shallow groundwater as reported previously for the present study area [8] . Ca− Mg− type water predominated in the samples based on our analysis. Ninety percent (90%) of the samples were categorized as Ca-Mg type. There are six main water supply zones in Dhaka City (Figure 3(c)); Zones 1 (leather industrial areas), Zones 1 and 2 are in the older more densely populated southern part of the city, Zone 4, 5 are the high traffic area, Zone 6 coincides with the major industrial district of Tejgaon located north of the old city center. Zones 1, 2 and 6 have seen the most intensive well development, but during the 1990s new drilling has increasingly extended to the other supply zones. Abundances of major ions and low dissolved oxygen (DO) 0.3 - 3.0 mg/L (Table 1) were found in Buriganga River water adjacent to industrial areas.
Color mapping was constructed to illustrate the relative abundances of the major anions (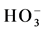 ,
, 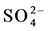 ,
, 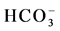 and Cl−) and cations (Na+, K+, Ca2+ and Mg2+) in water from different stations (Figure 3(d)). Most of the water samples tested lie in the range of
and Cl−) and cations (Na+, K+, Ca2+ and Mg2+) in water from different stations (Figure 3(d)). Most of the water samples tested lie in the range of 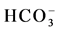 and can thus be considered as bicarbonate type water (Figure 3(e)). The color mapped data shows the variation between water from different sources and indicates the high concentration of major ions were found in water from industrial areas, especially Hazaribagh, as compared to water from another city (Bonani city) (Figure 3(d) and Table 1). The water from Buriganga River, Hazaribagh (stations: 1 - 13) and Tejgaon city (station: 20) had higher concentrations of minerals than other stations. The elevated minerals concentrations may be due to pollution of the discharge water from the industry (Figure 1(b) and Table 1). The color mapped data also shows the elevated cation and anion concentrations in the water samples obtained from stations in deep groundwater (stations, 2, 4, 9, 11, 13) as compared to young groundwater (station 7) and shallow groundwater (station 16) (Figure 2 and Figure 3(d)).
and can thus be considered as bicarbonate type water (Figure 3(e)). The color mapped data shows the variation between water from different sources and indicates the high concentration of major ions were found in water from industrial areas, especially Hazaribagh, as compared to water from another city (Bonani city) (Figure 3(d) and Table 1). The water from Buriganga River, Hazaribagh (stations: 1 - 13) and Tejgaon city (station: 20) had higher concentrations of minerals than other stations. The elevated minerals concentrations may be due to pollution of the discharge water from the industry (Figure 1(b) and Table 1). The color mapped data also shows the elevated cation and anion concentrations in the water samples obtained from stations in deep groundwater (stations, 2, 4, 9, 11, 13) as compared to young groundwater (station 7) and shallow groundwater (station 16) (Figure 2 and Figure 3(d)).
![]()
![]() (a) (b)
(a) (b)![]()
Figure 3. (a) Piper trilinear diagram of major ion and geochemical results for Dhaka tap water. Legend 1: Rock types are designed according to the domain in which they occur on the diagram segments. gypsum (1), calcite (2), dolomite (3), rhyolite (4), basalt (5), shale (6), seawater: 7, brine: 8. Legend 2: Classification diagram for anion and cation facies in the form of major-ion percentages; A-calcium type, B-no dominant type, C-magnesium type, D-sodium and potassium type, E-bicarbonate type, F-sulphate type, G-chloride type; (b) Compare the variation of ionic composition for different water bodies (rain, river and groundwater); (c) Water Levels Dhaka is zoned for water-supply purposes into six districts, with newer zones having the higher numbers; (d) Colored surface map of major ions distribution in rain, river, ground and tap water of the Dhaka Urban (unit: mg/L); (e) Dhaka Water are dominated by bicarbonate (Ca-HCO3 and Ca-Na-Mg-HCO3-Cl type).
In this study, four precipitation samples (Ra: 14, 15, 17, and 18) were collected at three sampling sites near high traffic area (Ra: 15, 18), high way and new building construction site (Ra: 17) and Buriganga River site (Ra: 14) (Table 1). NO3 was higher in rainwater.
HCO3-type water predominated based on anion analysis (99% of the samples), and the water was classified in one main group: bicarbonate (Figure 3(e)).
4.2. Dissolved Oxygen (DO) in Buriganga River
DO (mg/L) is considered in the assessment of Buriganga River water resources (not for drinking water) for the purposes of their sustainability to aquatic life or in wastewater treatment studies (Table 1). The DO level within the 17 km reach of the Buriganga is alarmingly low, at some locations it even goes down to 0.1 mg/L during the dry season [1] . The DO content in leather industrial discharge point is 0.3 mg/L (Table 1: R5).
4.3. Toxic Heavy Metals and Radioactive Isotopes in Dhaka Water
Toxic metal levels in river and groundwater samples in Dhaka (Figure 4(a), Table 2) show that the concentrations of heavy metals are quite different. Toxic (B, Se, Cr, Cu As, Pb, Cd, Mn, Fe) and radioactive elements (U238 and Cs133) were measured by using ICP-MS. The concentrations of radioactive elements (U238 and Cs133) were higher in industrial sites (Figure 4(b)). Elevated concentrations of Cr, B, Mn and Fe in Buriganga River water are usually due to leather industries [25] . In this study, the concentrations of Cr, B, Mn and Fe in the river water samples were 0.4 - 18.59, 49.58 - 138.87, 5.3 - 23.84, and 12.97 - 83.71 μg/L, respectively, whereas the concentrations of Cr, B, Mn and Fe in groundwater samples were 0.25 - 0.93, 35.99 - 143.93, 1.9 - 130.47, 0.25 - 0.93 (Table 2). The maximum contaminant level was evaluated with the standards limits for heavy metals in industrial wastewater, established by Japan Environmental Ministry are presented in Table 2 [26] . Concentration of arsenic (As) in Dhaka Water (ground and surface) shows minimum levels. Quantifying total arsenic (As) and As from interlocking geochemical cycles (Fe, Mn) may assist in interpreting As dynamics in Dhaka well water [26] . Fe and Mn minerals efficiently scavenge As [27] [28] . However, Buriganga River and adjacent groundwater contained high content of Cr and B [29] . Because, during the chrome tanning process. 50% unused chromium salts are usually discharged in the final efluents, causing a serious threat to the Buriganga River [4] . From these features it can be inferred that high content of major ions, trace metals (Cr) and other heavy metals are probably originate mainly from the wastewater and seem to be dumped to Buriganga River from leather industries. The highest level of heavy metals, boron (B), Cl, SO4, Na and EC were detected in Buriganga River and groundwater near leather industrial areas, suggesting common source for these metals in that area (Table 1 and Table 2). The discharged waste from leather industries contain high chemical levels including salinity, organic load, ammonia, sulfide, chromium, chloride, sodium and other salt and heavy metals etc. [30] . Shallow groundwater (station 16) adjacent to industrial areas is highly polluted by Zn,
![]() (a)
(a)![]()
![]() (b)
(b)
Figure 4. Colored surface map of heavy metals distribution in rain, river, ground and tap water of the Dhaka Urban (unit: µg/L). (a) Color map of toxic heavy metals; (b) Plot of radioactive metals in Buriganga River site (unit: µg/L).
Cu and Pb. The study revealed that anthropogenic elements were highly enriched (especially Zn, Pb, Cd) in rainwater (station 15, 17, 18) [31] . Significant portion of Zn and Pb, Se could be increased in air by the effect of local sources like vehicles, new building construction areas, respectively (Table 2). Zn and Cu concentration were very high in rain and groundwater at new building construction areas (St. 17, and 16). Pure metals or mixtures of metals such as Cu, Pb, and Zn are used in pipes, wiring, and roofing material and Pb come from high traffic and from industrial sources. Selenium remains in water as (selenate (![]() ) biselenite (
) biselenite (![]() ) selenite (
) selenite (![]() ), at pH 7.0 - 9.4 (WHO Guideline (1998)
), at pH 7.0 - 9.4 (WHO Guideline (1998)
![]()
Table 2. Toxic and trace metals in Dhaka water (rain, river and groundwater) (µg/L). Legend; R: river water, G: groundwater, Ra: rainwater. Legend; R: river water (R1, R3, R5, R6, R8, R10 and R12), G: groundwater (G2, G4, G7, G9, G11, G13, G16) and Ra: rainwater (Ra14, Ra15, G16, Ra17 and Ra18).
aJapan Limit 2012.
= 0.04 mg/L) [32] . Selenium concentration may increases at industrial areas (Stn.1 - 5) for the manufacturing operations.
4.4. Measurements of Water Isotope to Survey the Water Sources in Catchments and Recharge of δ18O in Groundwater
A plot of measured δ18O vs. δD identifies the mechanisms responsible for stream flow generation and can also identify the sources of solutes in contaminated systems.δ18Ο and δ2H follow the “meteoric water line Equation” in precipitation (rain, snow, sleet, hail, etc.):
![]() Eq. 2
Eq. 2
The parameter d, is known as “deuterium excess” (d-excess) (Dansgaard, 1964), which is characterized by a marked variation globally. However, a first estimate of the global average d-excess was originally reported by Craig (1961) to be 10‰ [33] .
The spatial distributions of δ18O and δ2H are shown in Figure 5(a) and Figure 5(b). The calculated ranges of isotopic compositions for Dhaka Water were −3.8‰ to −8.6‰ for δ18O values and −29.4‰ to −39.7‰ for δD values. The δ18O became depleted at the center of the city (Figure 5(c)) as well as in some contaminated Buriganga Water and increased in ground and river water in Buriganga areas (Figure 5(a), Figure 5(b)). This suggests that there may be some invasion of water from the Buriganga River. The δ18O increased in shallow gro- undwater (Saturia: −3.61% δ18O and −28.9‰ δD). Supply water from Balurpar, Gourlane, Nasirabad are belong to same sources, therefore, the δD and δ18O values and hexagonal diagram are similar for these 3 stations (Figure 5(d)). Although
Khilgaon is belong to the same area but the different δD and δ18O values and hexagonal shape revels that the supply water source is different compare to those 3 stations (Figure 5(d)). The δD vs δ18O diagram shown in Figure 5(d) indicates that the δD and δ18O values lie on the LMWL.
Therefore, the magnitude of the oxygen isotope shift depends on the original O-isotope compositions of both water and rock, the mineralogy of the rock, water contaminant, the water/rock ratio and the contact duration time [34] .
5. Conclusion
A number of geochemical dispersion patterns are obtained for major ions, trace heavy metals, isotopes (δ18O and δD) including radio isotope U238 in Dhaka Water. Chemical/isotopic monitoring was capable of discriminating between the effects of induced recharge from the polluted rain, Buriganga River in contaminated urban areas. The data of water tracer (δ18O and δD) indicated the water recharged from river to shallow groundwater system. Deep groundwater is not contaminated like shallow groundwater. The U238 map shows two prominent highs―both groundwaters are near the industrial areas. Maximum heavy metals (Cr, Pb, U238, Cs-133, etc.) and boron (B) show significantly higher concentrations in Buriganga River and groundwater near the leather industrial areas compared to other areas. High content of anthropogenic elements (Cu, Zn, Pb) was determined in areas, dominated by commercial and high traffic (Motijheel, Mirpur) and new building construction activity (Baribadh, Matikata, Dhaka). Water analyses also reveal a correlation between toxic heavy metals loading and its watershed; it hypothesizes that heavy metals in river and groundwater are directly correlated to heavy metals loading fluxes from direct discharge of wastewater from leather industries into Buriganga River, connecting with discharge drain near the Metro Housing, West Dhanmondi, Dhaka. The data for DO (0.3 - 3 mg/L) in Buriganga River agree that the hypoxic zone is the result of the huge loads of organic content that discharge from leather industries. As organic content decomposes, oxygen is consumed in the process, resulting in low levels of oxygen in the river water. This study provided important data information for better understanding geochemical condition in urban freshwaters. Although, Dhaka (central) soil has a large special capacity for adsorbing dissolved arsenic in the Bengal Aquifer system, the geochemical cycles are controlled by nonnative materials from anthropogenic sources that alter the Dhaka Geochemical system.
Acknowledgements
We want to give very special thanks to “Environmental Clean Project, Dhaka Bangladesh” for the purpose of local data collection, sampling and for Graphic Design.