1. Introduction
Characterisation of bioactive molecules is usually possible because of the versatile types of spectroscopic techniques and thus GC-MS has been demonstrated to be one of the valuable analytical tools for the analysis of mainly non-polar components and volatile natural products, e.g., mono- and sesquiterpenes [10] . The plants investigated in this paper include Aloe camperi, Meriandra dianthera and a Polyherb (prepared from the seeds of Lepidium sativum, Brassica nigra and Nigella sativa). The plants are widely used in the folk medicine for different purposes and are believed to have anti-diabetic activities. Moreover, the anti- diabetic activity of the plants, with the same extraction procedures, using in vivo model were previously reported. Thus, the crude methanol extracts of the plants were able to reduced blood glucose in Alloxan-induced diabetic rats [11] . The aim of the present study was to evaluate the in vitro effects of the crude methanol extracts and fractions of the plants using α-glucosidase and α-amylase enzyme inhibition activities. The in vitro anti-diabetic profile of Aloe camperi and Meriandra dianthera are presented for the first time. Moreover, the diverse compounds in the most bioactive non-polar fractions were identified and tentatively characterised using GC-MS analysis.
2. Materials and Methods
2.1. Chemicals and Reagents
Analytical grade solvents for the extraction, chromatography and in vitro studies were purchased from local chemical suppliers. Column chromatography was conducted on silica gel 60 (0.063 - 0.2000 mm, 70 - 230 mesh; Merck KGaA Germany) and thin layer chromatography (TLC) was performed using Silica gel 60 F254 (200 × 200 mm) plates (Merck KGaA, Germany). The α-amylse and α-glucosidase enzymes were purchased from the Sigma aldrich (St Louis, MO, USA).
2.2. Plant Materials
The fresh leaves of A. camperi and M. dianthera were collected from Adi- hawisha, Eritrea (15˚14'N 38˚58'E). The seeds of B. nigra, L. sativum and N. sativa constituting the Polyherb were collected from a herbal shop in Asmara, Eritrea. The plants were initially identified by their vernacular names and later validated by a plant taxonomist and voucher specimens were deposited in the Herbarium of Eritrea Institute of Technology (Voucher Numbers: 003-007/14). The leaves and seeds of the plants were washed with distilled water and dried under shade at room temperature. The dried leaves and seeds of the plants were powdered using an electric blender and passed through a sieve (0.50 mm) and saved until further use.
2.3. Extraction and Chromatographic Separation
Each powdered sample (100 g) was defatted three times using light petroleum (60˚C - 80˚C) for 4 hours and filtered using Whatman No.1 filter paper. The extract was discarded and thus the defatted plant materials were further macerated in 99.5% methanol (1000 mL) for three days with occasional stirring. The polyherb was prepared from equal proportion of the constituent seeds of the plants. The methanol extracts were evaporated in vacuo at 50˚C and the concentrated extracts were stored in air tight containers at 4˚C until further use. The extraction afforded 20.82, 28.84 and 21.14 g of A. camperi, M. dianthera and the Polyherb respec- tively. The crude methanol extracts were subjected to column chromatography. In each case, slurry of silica gel (150 g) was prepared using hexane and poured down carefully into a column; varying solvent combinations of increasing pola- rity were used as the mobile phase. Each sample was prepared in a ceramic mortar by adsorbing 8.0 g of the extract to 20 g of silica gel in methanol and dried on a hot plate. The dry powder was allowed to cool and then gently layered on top of the column. Elution of the extract was done with solvent systems of gradually increasing polarity using hexane, ethyl acetate and methanol. The collected fractions were analysed using TLC with various mobile phases pre- pared from hexane (Hex), ethyl acetate (EtOAc) and methanol (MeOH) were used and gave good separation of the components of the crude mixture. The following combinations―Hex:EtOAc (8:2), Hex:EtAOc:MeOH (10:4:1) and Hex:EtOAc:MeOH (5:8:3) were mainly used and thus gave good separation of the components. The TLC plates were observed using a UV-VIS before and then after colour development by passing iodine fumes. Based on the Rf values, simi- lar fractions were pooled together and seven different fractions for each plant were screened using the in vitro anti-diabetic assays.
2.4. In Vitro α-Amylase Inhibition Assay
The assay was performed with slight modification of a previously reported me- thod [12] . Different concentrations (25 - 800 μg/mL) of the plant extracts and standard acarbose were prepared in dimethyl sulfoxide from 1 mg/mL stock solution. Each extract and standard (500 μL) were added to a 0.5 mg/mL α-amy- lase solution (500 μL) and incubated for 10 min at room temperature. Then 1.0% starch solution (500 μL) was added and incubated for another 10 min at room temperature. After that the colouring reagent, dinitrosalicylic acid (DNS, 1 mL) was added to the reaction mixture and heated in a boiling water bath for 5 minutes. After cooling, it was diluted with 10 mL of distilled water. The absorbance was measured at 540 nm against the blank reagent using a double-beam UV-VIS spectrophotometer (UV-1800; Shimadzu). The percentage enzyme inhibition activity of the extracts/standard was calculated using the following formula shown in Equation (1).
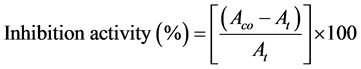 (1)
(1)
Where, 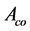 is absorbance of the control and
is absorbance of the control and 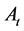 is absorbance of the test samples.
is absorbance of the test samples.
Control incubations represent 100% enzyme activity and were conducted in a similar way by replacing extracts with the vehicle (25 μL dimethyl sulfoxide and distilled water). For blank incubation, in order to allow for the absorbance produced by the extract, the enzyme solution was replaced by the buffer solution and the absorbance was recorded. Separate incubation carried out for reaction t = 0 was performed by adding samples to DNS solution immediately after addition of the enzyme.
2.5. In Vitro α-Glucosidase Inhibition Assay
The assay was performed with slight modification of previously reported method [13] . From 1 mg/mL stock solution different concentrations (25 - 800 μg/mL) of the plant extracts were prepared in 5 % dimethyl sulfoxide. Each extract and standard (500 μL) were added to a 1 U/mL of α-glucosidase (500 μL) and then incubated for 5 minutes at room temperature. Then 37 mM maltose solution (500 μL) was added and incubated for 30 minutes. After that the glucose oxidase/peroxidase kit reagent (1 mL) was added to the reaction mixture and kept aside for 15 minutes. Tris buffer (1 mL) was then be added to the mixture. The absorbance was measured at 540 nm against the blank reagent. The α-glucosidase inhibition was expressed as a percentage of inhibition.
2.6. GC-MS Conditions
The fractions were analysed using a Thermo (Hemel Hempstead, UK) Trace GC-MS equipped with a single quadrupole analyser. Gas chromatography was undertaken via a J & W Innowax 60 m × 0.25 mm 0.5 µm thickness polar column using helium as a carrier gas at 1.0 mL/min. The injector temperature was set at 240˚C and samples were injected in split less mode. Low resolution mass spectra were recorded over a mass range of m/z 20 - 500 using a 70 eV electron ionisation. Identification of the compounds was performed in com- parison with database (NIST08 Library). The working conditions of the GC-MS are furnished in Table 1.
2.7. Data Analysis
The in vitro experiments were performed in triplicate and the results were recorded as the mean ± SD. Statistical difference between the control and treatment groups was determined using ANOVA followed by Tukey’s test for post hoc analysis. P ≤ 0.05 was considered as significant. The median inhibitory concentration (IC50 values), defined as the concentration of the samples causing 50% inhibition, were determined by linear and non-linear regression plots with varying concentrations of the plant extracts against percentage inhibition (Table 2).
3. Results
3.1. In Vitro Assay of Crude Extracts
The in vitro screening of all the crude extracts has generally elicited dose
![]()
Table 1. Instrumental conditions of the GC-MS.
![]()
Table 2. Regression analysis equations (with R2*) used for the IC50 calculations.
*R2 are indicated inside the brackets
dependent α-glucosidase and α-amylase inhibition activities. The highest con- centrations (0.800 mg/mL) of the extracts and Acarbose showed maximum inhibitions, while the lowest concentrations (0.025 mg/mL) showed minimum inhibitions relatively. A maximum of 87.8%, 63.5%, 58.7% and 55.3% inhibition of α-glucosidase activity was observed at 0.800 mg/ml concentration of the crude extracts from M. dianthera, A. camperi, Polyherb and Acarbose, respectively. As indicated in Figure 1(a), M. dianthera displayed the highest α-glucosidase inhi- bitory activity (IC50: 0.074 ± 0.032 mg/mL) at the highest concentration tested relative to A. camperi, the Polyherb and Acarbose (IC50: 0.37 ± 0.052, 0.56 ± 0.024 and 0.55 ± 0.029 respectively). A. camperi and the Polyherb showed similar α-glucosidase inhibitory activities as compared to Acarbose. This indi- cates that the extracts are very potent α-glucosidase inhibitors in comparison with acarbose. The α-glucosidase inhibition activities of A. camperi and M. dian- thera, except for the Polyherb and Acarbose, were significantly different (P < 0.05) at various ranges of concentrations (0.025 - 0.800 mg/mL). On the other hand, the percentage α-amylase inhibitions of M. dianthera, A. camperi, the Polyherb and Acarbose, at the highest concentrations (0.800 mg/mL), were 78.3%, 15.9%, 16.4%, and 82.9% respectively. As shown in Figure 1(b), A. camperi and the Polyherb displayed lower α-amylase inhibitory activities (IC50: 1.72 ± 0.06 and 2.57 ± 0.07 mg/mL respectively) compared to Acarbose and M. dianthera (IC50: 0.31 ± 0.01 and 0.43 ± 0.02 mg/mL respectively). For the α-amylase inhibition activity, even at lower concentrations (0.025 mg/mL), there were statistically significant differences (P < 0.01) among the values generated for all the extracts (Figure 2). Moreover, there was high statistical difference (P < 0.001) among the percentage inhibition of the groups at higher concentration (0.800 mg/mL).
3.2. In Vitro Assay of Fractions
The α-amylase and α-glucosidase inhibition activities of the fractions prepared from the three plants of interest are shown in Figure 2. The in vitro anti-diabetic activities of the fractions (0.800 mg/ml) were evaluated in the same methods as the crude samples. With some exceptions, most of the fractions displayed characteristic enzyme inhibition activities. The non-polar fractions of A. cam- peri (A1, A2), M. dianthera (M2) and Polyherb (P1) and the moderately polar fractions of M. dianthera (M4, M5) and Polyherb (P3, P4) elicited relatively higher α-glucosidase inhibitory activities. However, the most polar fractions of M. dianthera (M6) and the Polyherb (P6, P7) showed no inhibitory activities against α-glucosidase. Similarly, the non-polar fractions labelled as A1, M1, P1 and P2 displayed higher α-amylase inhibition activities as compared to the polar fractions. As shown in Figure 2, the α-glucosidase activities were not signifi- cantly different among the polar and non-polar fractions. The moderately polar fraction labelled as M3 and the most polar fractions labelled as M6, P6, and P7 showed no inhibitory activities against α-amylase. The non-polar fractions A1, M1 and P1 displayed statistically significantly different α-amylase inhibitory activities (P < 0.01, P < 0.001 and P < 0.01 respectively) than their corresponding fractions. Generally, the non-polar fractions exhibited better α-glucosidase and α-amylase inhibitory activities and thus were subjected to GC-MS analysis in order to identify and characterize the bioactive metabolites responsible for the reported activities. Similar results of α-amylase and α-glucosidase inhibition were reported from Taxus sumatrana of Japan; the non-polar (hexane) extract of the plant showed the highest enzyme inhibitory activity and on characterization afforded bioactive fatty acid methyl esters [14] .
![]() Results reported as mean ± SD (n = 3); ***P ≤ 0.001, ** P ≤ 0.01, *P ≤ 0.50 and NSP > 0.50 (non-significant) relative to the positive control (Acarbose).
Results reported as mean ± SD (n = 3); ***P ≤ 0.001, ** P ≤ 0.01, *P ≤ 0.50 and NSP > 0.50 (non-significant) relative to the positive control (Acarbose).
Figure 1. The α-glucosidase and α-amylase inhibitory activities of the crude extracts.
![]() Results reported as mean ± SD (n = 3); A1-A7: fractions from A. camperi, M1-M7: fractions from M. dianthera and P1-P7: fractions from Polyherb *** for P < 0.001, ** for P < 0.01 and NS for not-significant (Statistical significance was based on comparison to most of the other fractions).
Results reported as mean ± SD (n = 3); A1-A7: fractions from A. camperi, M1-M7: fractions from M. dianthera and P1-P7: fractions from Polyherb *** for P < 0.001, ** for P < 0.01 and NS for not-significant (Statistical significance was based on comparison to most of the other fractions).
Figure 2. Comparison of the enzyme inhibitory activities of the fractions.
3.3. GC-MS Analysis of Fractions.
The non-polar fractions (hexane-ethyl acetate; 9:1, 8:2, 7:3) with the highest inhibitory activities were analysed using GC-MS. Based on the GC-MS analysis of the TIC of M. dianthera (Figure 3), the most active non-polar fractions presented various essential oils including camphor, terpineol, pinocarveol, verbenol, caryophyllene oxide, ledol, isolongifolene, borneol, spathulenol, thymoquinone, β-eudesmol and thymol. Generally, several essential oils found in plants were reported to have anti-diabetic activities [15] . Thymol, a monoterpene phenol, exhibited promising anti-diabetic activity [16] and isolongifolene, a sesquiterpenes, was reported to have antioxidant properties which might be helpful to prevent the progress of oxidative stress related to diabetes [17] . β-Eudesmol, a sesquiterpenoid alcohol, potentiates the neuromuscular blocking effect in phre- nic nerve-diaphragm muscle preparations of normal and alloxan-diabetic mice [18] . Moreover, bioactive fatty acid methyl esters (FAMEs) including palmitic acid methyl ester, α-linolenic acid methyl ester and other benzene derivatives were characterized in M. dianthera. Some FAMEs were reported to have anti- diabetic related activities [19] and palmitic acid was reported with potential α- amylase inhibitory activity [20] . Representative GC-spectra of the non-polar fractions (M1 and M2) of M. dianthera and the list of compounds found in the most active non-polar fractions are displayed in Figure 3 and Table 3 respec- tively.
![]()
![]()
Figure 3. GC-MS total ion chromatograms of the non-polar fractions of M. dianthera.
![]()
Table 3. List of compounds found in the most active non-polar fractions of M. dianthera.
M1 and M2 represent fraction 1 and fraction 2 of M. dianthera respectively; RT―retention time, SI―simi- larity index.
As shown in Figure 4, the two non-polar fractions of A. camperi showed characteristic peaks representing various compounds. The most active non-polar fractions of A. camperi offered bioactive benzoic acid derivatives including 2- hydroxy-benzoic acid ethyl ester (ethyl salicylate) and 2-hydroxy-benzoic acid phenyl methyl ester (benzyl salicylate). Benzoic acid and its derivatives were reported to have antioxidant and anti-diabetic properties [21] . A study undertaken clarified the carboxyl group as being essential to the salicylate structural requirements for hypoglycemic activity [22] . Other compounds found in A. camperi include bioactive compounds including palmitic acid methyl ester, palmityl alcohol and 10-undecenoic acid methyl ester (Table 4). Thus, the reported bioactivity of the non-polar fractions of A. camperi could be due to the benzoic acid derivatives and FAMEs.
Mostly FAMEs were profiled in the most active non-polar fractions of the Polyherb (Table 5). The bioactive FAMEs include myristic acid methyl ester, palmitic acid methyl ester, palmitoleic acid methyl ester, stearic acid methyl ester, oleic acid methyl ester, linoleic acid methyl ester and α-Linolenic acid methyl ester. FAMEs including oleic acid and α-linolenic acid were identified as the potential biomarkers of T2DM for their powerful discriminant ability of T2DM patients from healthy controls [23] . Addition of oleic acid in diet in Type 2 diabetic patients reduced insulin resistance and restored endothelium-dependent vasodilatation [24] and oleic acid was found to be effective in reversing the inhibitory effect in insulin production of the inflammatory cytokine tumor necrosis factor alpha (TNF-α) [25] . Studies have also shown that omega-3 fatty acids such as α- linolenic acid offer a direct or indirect reno-protective effect in diabetes patients [26] . Moreover, conjugated fatty acids like linoleic acid lower insulin resistance which may help prevent adult-onset of diabetes [27] . Therefore, the observed bioactivity of the non-polar fractions of the Polyherb is mainly due to the FAMEs. The spectra of the two non-polar fractions of the Polyherb are shown in Figure 5.
4. Discussion
The enzyme inhibitory activities have been adopted as reliable techniques of evaluating the anti-diabetic potential of different medicinal plants and thus inhi-
![]()
![]()
Figure 4. GC-MS total ion chromatograms of the non-polar fractions of A. camperi.
![]()
Table 4. List of compounds found in the bioactive non-polar fractions of A. camperi.
A1 and A2 represent fraction 1 and fraction 2 of A. camperi respectively.
![]()
Table 5. List of compounds found in the most active non-polar fraction of the Polyherb.
P1 and P2 represent fraction 1 and fraction 2 of the Polyherb respectively.
bition of the action of the digesting enzymes prolong overall carbohydrate digestion time by delaying its breakdown, causing a decrease in the rate of glucose absorption and consequently blunting the post prandial rise in plasma glucose [28] . From the results, it is evident that the crude methanol extracts of the three plant preparations displayed potential inhibitory properties against the α-amylase and α-glucosidase enzymes activities which are comparable to the commercial drug (Acarbose). Acarbose is a known α-amylase and α-glucosidase inhibitor, currently used in anti-diabetic therapy for reducing postprandial increase in
![]()
![]()
Figure 5. GC-MS total ion chromatograms of the non-polar fractions of the Polyherb.
blood glucose levels [29] . Therefore, the crude plant extracts proved to have effective anti-diabetic activities and thus the observed results complement the traditional use of the plants in the management of diabetes. Moreover, the in vitro results reported in this paper are supplementary to the published anti-diabetic activities of the plants investigated using the in vivo assays [29] .
GC-MS has been employed in the analysis of FAMEs and it is particularly advantageous for FAME determination in complex biological samples, where spectrometric confirmation of analytes is advisable. The high chromatographic resolution of GC permits separation of structurally similar fatty acids and provides greater sensitivity that would be very difficult to separate by HPLC [30] . In this study, GC-MS has proved to be a better separation and characterisation of
![]()
Figure 6. Electron ionization mass spectra of certain FAMEs present in the fractions.
the FAMEs; similar finding was reported before [31] . Generally, the mass spectra of most saturated FAMEs exhibited the presence of corresponding [M]+, [M − 31]+ and [M − 43]+ peaks, as well as intense peaks at m/z 74, 87 and 143, which are characteristics of saturated FAMEs [32] . As shown in Figure 6, the spectra of some of the FAMEs listed in Table 5 displayed the characteristic peaks which are used for identification of the compounds. The efficacy of medicinal plants is usually a product of the synergic effects of several natural compounds and therefore, the observed anti-diabetic activities of the non-polar fractions of the plants can be attributed to the presence of the bioactive essentials oils, benzoic acid derivatives and FAMEs.
Acknowledgements
The authors would like to thank Japan international cooperation agency (JICA) for providing the financial support. We also thank Department of Chemistry of the University of Southampton for the GC-MS analysis of the samples.