Reduction in Water Stress for Tree Saplings Using Hydrogels in Soil ()
1. Introduction
The successful establishment and maintenance of urban trees are often seriously constrained by water stress. Urban trees are exposed to excess and deficit soil moisture, but drought is generally considered to be the more serious health threat. Impermeable surfaces and compacted soil diminish infiltration of precipitation into tree root zones [1] . Also, urban soils are warmer than soils in natural areas because of the surrounding pavement and lack of vegetation [2] . Lack of adequate precipitation infiltration and the high temperature of urban soil can result in drought stress which will limit plant growth and function through a series of morphological, physiological and metabolic changes [3] . Plants close their leaf stomata in response to moderate water stress [4] , which decreases leaf transpiration [5] . Subsequently, the diffusion of CO2 from the atmosphere into carboxylation sites in leaves decreases and photosynthesis is impeded [6] . As the water stress worsens, photosynthetic activity decreases, mainly due to chloroplast dehydration and the consequent biochemical constraints [4] . Ionic, osmotic, or other effects of cellular water loss can inhibit biochemical processes in water- stressed plants [7] .
Several studies demonstrated a decline in the chlorophyll content of plants under water stress [3] [4] [8] , so chlorophyll content could be a useful indicator of drought stress. As a surrogate measure of the vegetation vigor and productivity of plants, normalized difference vegetation index (NDVI) is another estimator of plant response to water deficit [9] . This index is calculated from the red (RRed) and near-infrared (RNIR) reflectance from vegetation (Equation (1)).
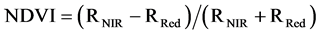 (1)
(1)
The NDVI ranges from −1 to 1, with 0 representing no vegetation. Negative values represent non-vegetative surfaces, while values approaching 1 represent very dense vegetation [10] . Aguilar et al. [11] found that annual changes in the NDVI of woody plants were associated with changes in precipitation and groundwater. They measured lower NDVI values (0.65 - 0.70) in the dry years and found linear relationships between accumulated rainfall and maximum NDVI values as indicators of overall productivity of plants. Over a nine-year period (1989-1997), Wang et al. [12] also found a positive correlation between the NDVI and the soil moisture values in Kansas forestlands.
1.1. Super Absorbent Polymers or Hydrogels
There have been few studies of water saving technologies in urban environments, especially involving tree pits. A technology to potentially reduce the effect of drought stress, particularly on seedlings and saplings, could be the hydrophilic, cross-linked polymers known as super absorbent polymers or hydrogels. The term hydrogel is sometimes used for these compounds because when the dry crystals absorb water, they take on the consistency of a gel [13] . Hydrogels can retain up to 400 times their weight in water when saturated, but will not dissolve in water [14] . The hydrophilic functional groups attached to the polymeric backbone enable hydrogels to absorb water, while the cross-links between network chains make hydrogels resistant to dissolution [15] . Ideally, at least 95% of the water stored in hydrogels is available to plants [16] .
Hydrogels can also retain nutrients when incorporated into the soil, making them available for plant growth whenever required. Hydrogels are hydrophilic and contain carboxylic groups, enabling them to bind cations and adsorb water [17] . The water and nutrients stored in hydrogels are released gradually for plant growth under water limiting conditions, whereas under non-water limiting conditions, they are reported to enhance nutrient uptake for plant growth [18] . This is of special interest for urban foresters, because water stress and nutrient deficiency are two important growth-limiting factors for street trees.
Different factors affect the absorptive capacity of hydrogels for water. These factors include the tolerance of hydrogels to ionic solutions, the tensions at which they bind water, and the speed with which they degrade in the field [19] . According to Orzolek [20] , under field conditions, polymers will lose 10% to 15% of their activity each year. The degradation of polymers can be due to microbial activity [21] , chemical decomposition [20] , or physical modification over time. In spite of the beneficial aspects of hydrogels in the soil and their effects on plant growth, an overabundance of hydrogels can have negative consequences in some cases [13] . For instance, Sarvaš et al. [22] observed high mortality (64%) of pine seedlings caused by an overdose of hydrogel. Shooshtarian et al. [23] attributed a high seedling mortality rate in hydrogel-amended soil to a reduction of free air space resulting from the swelling of the hydrogel and the consequent reduction in soil aeration.
In general, the main function of hydrogel in soil is to preempt the negative effects of water deficiency on plant growth and productivity. However, its effectiveness varies depending on the situation. Accordingly, the objective of this research was to observe the effects of differential proportions of hydrogel, under conditions of different water availability, on the growth of Siberian elm and silver maple in greenhouse experiments.
2. Materials and Methods
2.1. Growth Test
The project was performed in a greenhouse located on the Macdonald Campus of McGill University, Sainte-Anne-de-Bellevue, Canada, under humid summer conditions of 28˚C - 30˚C, 65% relative humidity, and a 16-h photoperiod. The experiment was run in two phases of six weeks each: the first from Aug. 18th to Sep. 30th, 2014, and the second from Oct. 2nd to Nov. 11th, 2014. Siberian elm (Ulmus pumila) and silver maple (Acer saccharinum) saplings were potted in sieved soil (tamisé) (Laniel Prodamex, Pierrefonds, QC). The soil in each pot was thoroughly mixed with one of two proportions (0.5 and 1.0% dry weight) of dry Super ABA 200 (Iramont Inc., Laval, QC), a potassium polyacrylamide hydrogel, as compared to the control soil (0% of hydrogel). Water-soluble fertilizer (20-20-20, Passion Jardins, Vaudreuil-Dorion, QC) was added to the soil at the beginning of the experiment.
In the first phase of the experiment, the trees were arranged in a full-factorial, completely randomized design with twelve replicates, totaling 72 pots. The trees were irrigated regularly and the pots were randomly rearranged at each watering. At the end of this phase, 18 trees, i.e. three replicates per treatment, were sacrificed and analyzed for growth and physiological parameters.
The second phase used the remaining trees (Siberian elm and silver maple, potted in soil with one of three proportions of hydrogel), but also included three different irrigation schedules, replicated three times, totaling 54 trees. The trees were either watered daily (no stress), weekly (moderate stress), or every second week (severe stress). In order to keep the water availability similar within treatment levels, the same amount of water was added to each pot in that level at the time of watering.
2.2. Growth and Physiological Measurements
The measured growth parameters were total height from the soil surface to the top of the crown and trunk diameter at the soil surface, measured every second week. Normalized difference vegetation index (NDVI) was measured using a Crop Circle™ ACS-430 active crop canopy sensor (Holland Scientific, Lincoln, NE). Leaves were tested using a SPAD 502 Plus Chlorophyll Meter (Spectrum Technologies, Aurora, IL). The SPAD-502 meter measures leaf transmittance in the red (650 nm) and infrared (940 nm) bands. The SPAD meter value, typically between 0.0 and 50.0, is proportional to the amount of chlorophyll in the leaf [24] . Both NDVI and SPAD readings were measured and recorded every second week.
2.3. Statistical Analysis
A Kolmogorov-Smirnov test showed that none of the data were normal, so a two-step transformation was used to rank the values by percentile, giving uniformly-distributed probabilities, and then map them onto normally-distributed z-scores using an inverse-normal function [25] . A general linear model was performed to diagnose the significant interactions between independent variables (hydrogel concentration, watering frequencies and the species). Analysis of Variance (ANOVA) was then applied to the transformed data to test for significant effects of tree species, hydrogel concentration, and watering frequency on the transformed values of growth, NDVI, and SPAD readings (α = 0.05) (SPSS Statistics v.21, IBM Corporation, Armonk, NY). Mean values of the measured variables for each hydrogel concentration were compared among watering frequencies using the Tukey method (α = 0.05) [26] . A bivariate correlation test was also performed between the dependent variables (stem height, stem diameter, NDVI, and SPAD readings) and the independent variables (watering regime and hydrogel concentration).
3. Results and Discussion
3.1. Patterns of Growth and Photosynthetic Activity under Different Watering Regimes
Hydrogel had no observable effect on well-watered trees, as measured during the first phase of the experiment. This is consistent with previous studies [27] . The experimental factors did have significant effects on the water-stressed trees, as observed during the second phase of the experiment. Significant interactions were observed between tree species and hydrogel concentration, and between tree species and watering frequency (Table 1). This result indicates that the elms and maples each responded differently to the hydrogel treatment and watering frequency. Therefore, the rest of the statistical analysis was performed separately on the two tree species.
3.2. Stem Height and Diameter
According to Rais et al. [28] , drought-induced water stress can influence tree growth even before it has an evident effect on physiological processes such as photosynthesis. We observed that change in stem height was a more sensitive indicator of drought stress than stem diameter in both species (Table 2), as have other authors [28] . Bouriaud et al. [29] surmised that tree height is sensitive to water stress because of restricted movement of water to the actively growing extremities. Moreover, water stressed trees might favor root extension over crown expansion in an attempt to compensate for the water deficit [28] [30] .
We observed a negative effect of watering frequency on the height of silver maple (r = −0.29, p < 0.05). In corroboration with this result, Elhadi et al. [31] also found tree height increased more rapidly when saplings were watered every nine days than when they were watered every three or six days. They concluded that frequent irrigation is not always profitable for plant growth because it can negatively affect root respiration and growth. As a result, water and nutrient uptake by roots diminishes and tree growth declines [32] .
Under water stress, 0.5% hydrogel concentration increased stem height in both tree species (r = 0.48, p < 0.05, Figure 1, Table 2 and Table 3). We also observed a significant interaction between hydrogel and watering frequency on
![]()
Table 1. Significance of interaction of species, SAP weight percentages, and watering frequency on growth and physiological parameters.
***Significant at p ≤ 0.001; **Significant at p ≤ 0.01; *Significant at p ≤ 0.05.
![]()
Table 2. The significance of SAP, watering frequency and between subject effects on stem height and diameter.
***Significant at p ≤ 0.001; **Significant at p ≤ 0.01.
![]() (a)
(a)![]() (b)
(b)
Figure 1.Comparison mean effect of SAP levels (wt%) on stem height at different watering frequency for (a) Siberian elm and (b) silver maple. Values are the mean (±standard error) of the replicates per treatment.
![]()
Table 3. Significance of mean difference of growth and physiological parameters for Siberian elm and silver maple based on SAP weight percentages.
***Significant at p ≤ 0.001; **Significant at p ≤ 0.01; *Significant at p ≤ 0.05.
stem height, indicating that the effect of the hydrogel differed depending on the watering regime (Table 2). Various authors have attributed the positive effects of hydrogel on plant height and survival during drought conditions to improved root growth or to the retention of soil water near the newly planted seedling [27] .
3.3. Normalized Difference Vegetation Index (NDVI)
Physiological processes in plants are highly constrained by water deficits [33] , as illustrated by the positive correlation between NDVI and watering frequency both for Siberian elm (r = 0.37, p < 0.01) and for silver maple (r = 0.16, p < 0.05). Under drought stress, stomata tend to close, the rate of photosynthesis decreases, and respiration increases [34] . Hassan and Ali [35] and Silber et al. [36] suggested, however, that reduced photosynthesis during drought can be due to nutrient deficiency rather than lack of water. In either case, hydrogels can absorb either water or nutrients from the soil [37] and gradually release them to the soil in the root zone during drought conditions [38] . The absorption and rerelease of nutrients by the hydrogel can maintain photosynthetic rates [39] . This might explain the positive correlation of hydrogel with NDVI (0.5%, r = 0.25, p < 0.01) in both elms and maples (Figure 2 and Table 3 and Table 4), while the interaction between hydrogel and watering frequency did not have a significant effect on NDVI for the maples (Table 4).
3.4. SPAD Readings
The effect of watering frequency on SPAD readings (chlorophyll content) was not significant in this study. Other authors considered invariance of chlorophyll content and photosynthesis under stress as physiological resilience [40] [41] .
Under drought stress, hydrogel concentration was negatively correlated with SPAD readings for elms (hydrogel = 1%, r = −0.37, p < 0.01, Figure 3(a), Table 3). Others have also reported that hydrogel had a slightly negative or null effect on plant performance [42] . However, the concentration of hydrogel was positively correlated with SPAD readings (chlorophyll content) in the maples (hydrogel = 1%, r = 0.62, p < 0.01, Figure 3(b)). The different responses of the two tree species are consistent with their coping strategies in the face of water stress [18] [43] . In general, silver maples grow in wet, poorly drained soils as in floodplains or stream banks [44] [45] . They are tolerant of long periods of inundation and oxygen deficiency [46] . On the contrary, Siberian elms prefer well-drained soil [47] . As the concentration of hydrogel in the soil increases, free airspace and oxygen availability can be reduced when the hydrogel swells. Silver maples are well adapted to such conditions while Siberian elms are not.
4. Conclusion
The addition of hydrogel to the potting soil had a significant effect on the growth, photosynthesis, and chlorophyll concentration of Siberian elm and silver maple saplings under water stress. In water-stressed trees, 0.5% (dry weight)
![]() (a)
(a)![]() (b)
(b)
Figure 2. Comparison mean effect of SAP levels (wt %) on NDVIs at different watering frequency for (a) Siberian elm and (b) silver maple. Values are the mean (±standard error) of the replicates per treatment.
![]()
Table 4. Significance of SAP, watering frequency and their interaction effects on NDVI and SPAD readings.
***Significant p ≤ 0.001; **Significant at p ≤ 0.01; *Significant at p ≤ 0.05.
hydrogel increased growth in both tree species, as indicated by change in height, and also increase in NDVI (photosynthesis) (p < 0.01). These effects were presumably due to the ability of hydrogel to absorb and rerelease nutrients and water. There was also a species-specific relationship between the concentration of hydrogel in the soil and the SPAD readings (chlorophyll content) of Siberian elms and silver maples. The specificity of this effect might be attributed to differences in the adaptation of the tree species to water stress. Since Siberian elm
![]() (a)
(a)![]() (b)
(b)
Figure 3. Comparison mean effect of SAP levels on SPAD readings at different watering frequency for (a) Siberian elm and (b) silver maple. Values are the mean (±standard error) of the replicates per treatment.
and silver maple are popular urban tree species in temperate climates, it is recommended that they be evaluated in field trials using hydrogel-amended soils, as a possible countermeasure to the water stress, which is associated with urbanization and climate change. A relatively high proportion of impermeable surfaces in urban environment and change in the intensity and frequency of rainfall due to the climate variability result in water stress for street trees and increase the need for improving the capability of urban soil to deal with water stress and buffer its effect for street trees with soil amendments such as hydrogel.
Acknowledgements
This project was financially supported by NSERC Discovery Grant program. The authors also thank Mr. Guy Rimmer for providing the greenhouse services available for the research team.