Cyclocondensation Reactions of Hydrazonoyl Chlorides with Some Azines: Synthesis of New Fused Heterocycles of Expected Microbiological Activity ()
1. Introduction
The interest in the chemistry of hydrazonoyl halides is a consequence of the fact that they undergo a wide variety of reactions which provide routes to a number of both heterocyclic and acyclic compounds [1] [2] [3] [4] . In addition, diverse biological activities such as anthelmintic, antiviral, antimicrobial, pesticidal, etc., have been found to be associated with hydrazonoyl halides. In recent years, interest to the chemistry of this class of compounds has been renewed because of the development of novel synthetic routes and their use as versatile synthon in synthesis of other compounds that found many applications in both industrial and pharmaceutical fields [5] - [19] . In continuation of our studies dealing with the utility of hydrazonoyl halides for synthesis of various heterocycles [3] [16] [20] [21] [22] , we wish to report herein a new facile synthesis of 1,3,6,9,11-pen- tasubstituted pyrido[3,2-f:6,5-f']bis([1,2,4]triazolo[4,3-a]-pyrimidin-5(1H)-ones (6) and 1,3-disubstituted-7-[(E)-2-(thiophen-2-yl)ethenyl]-1,4,9,9a-tetrahydro- 6H-[1,2,4]triazino[4,3-b] [1,2,4,5]-tetrazin-6-ones (16) that have not been reported hitherto, via cyclocondensation reactions of hydrazonoyl chlorides with some azines. Our interest in exploring a new simple synthetic strategy for the latter ring system is due to the fact that literature search reveals that the design and synthesis of novel mono-, di-, and poly-cyclic fused nitrogen heterocyclic compounds are among the active principles in chemical materials, particularly those displaying strategic roles in the development of different industries, especially from the biological point of view. Development of heterocycles with antimicrobial and antitumor activities represents one of the most important researches in therapeutical chemistry [23] .
Some fused heterocycles showed antibacterial, antifungal [24] , antiphlogistic, antitumor [25] , herbicidal [26] and in vitro antiviral and antitumor activities [27] .
2. Material and Methods
2.1. Chemistry
All melting points were measured on an electrothermal melting point apparatus and are uncorrected. The 1H and 13C NMR spectra were recorded in deuterated dimethyl sulphoxide (DMSO-d6) at 300 MHz on a Varian Mercury VXR-300 NMR spectrometer (Cairo University, Egypt). 600 MHz Bruker NMR spectrometer (King Abdelaziz University, Saudi Arabia) and chemical shifts were related to that of the solvent. The infrared spectra were recorded in potassium bromide discs on a Pye-Unicam, SP300 and Shimadzu, FT IR 8101 PC infrared spectrophotometers.
Biological activity was carried out at the Microanalytical Center of Cairo University, Cairo, Egypt. Mass spectra were recorded on a Shimadzu GC MS-QP 1000 EX mass spectrometer at 70 e.V. Elemental analyses were carried out at the Microanalytical Center of Cairo University, Cairo, Egypt. The starting compounds, N-phenyl benzenecarbohydrazonoylchloride (1a) [28] [29] [30] , N-phenyl-2-phenylami-no-2- oxoethanehydrazonoyl chloride (1b), N-phenyl-2-oxopropanehydrazonoyl chloride (1c), ethyl N-phenylhydrazonochloroacetate (1d) and ethyl N-p-nitro-phenylhy- drazonochloroacetate (1e) [20] [31] were prepared as previously reported.
Reaction of N-Phenyl benzenecarbohydrazonoyl chloride (1a) with 6- aminouracil-2-thione (2): Synthesis of 7-amino-1,3-diphenyl [1,2,4]triazolo [4,3-a]pyrimidin-5(1H)-one (3) [21] .
To a mixture of 6-aminouracil-2-thione (2) [32] (2.8 g, 20 mmol) and N- phenyl benzenecarbohydrazonoyl chloride (1a) (4.6 g, 20 mmol) in 50 ml dioxane (and 10 ml DMF for solubility reasons), 2.8 ml (20 mmol) of triethylamine were added, and the mixture was heated under reflux until H2S evolution ceased (15h).Then the solvent was distilled off, and the residue was cooled. The solid formed was filtered, washed with methanol and crystallized from ethanol : dioxane (v/v, 3:1) to give 7-amino-1,3-diphenyl[1,2,4]triazolo[4,3-a] pyrimidin- 5(1H)-one (3), yield: 2.29 g (38.6%); m.p. 260˚C - 262˚C (Lit. m.p. 262˚C - 264˚C [21] ).
Reaction of 7-Amino-1,3-diphenyl[1,2,4]triazolo[4,3-a]pyrimidin-5(1H)- one (3) with benzaldehyde and ethyl cyanoacetate: Synthesis of 8-amino- 1,3,6-triphenyl-7-ethoxycarbonyl-pyrido[2,3-f][1,2,4]triazolo[4,3-a]pyrimidin-5 (1H)-one (4).
7-Amino-1,3-diphenyl[1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one (3) (2 g, 6.6 mmol) was dissolved in 40 ml of dimethylformamide. To this solution, 0.8 ml (6.6 mmol) benzaldehyde and then 0.8 ml (6.6 mmol) ethyl cyanoacetate were added. The mixture was refluxed for 6 h. After cooling, the solvent was evaporated and 20 ml H2O cold was added. The solid formed was filtered, washed with cold H2O and then crystallized from ethanol:dioxane (v/v, 9:1) to give 4.
Yield: 3.3 g (99.69%); m.p. 297˚C - 300˚C; IR (KBr):  = 3348, 3164 (NH2), 1671, 1634 (CO) cm−1; 1H NMR (CDCl3): δ,1.25 (t, 3H, CH3), 4.31 (q, J = 7.3, 2H, CH2), 6.75 (s, 2H. NH2, D2O exchangeable), 7.20 - 7.80 (m, 15H, Ar-H); 13C NMR (DMSO-d6, 75 MHz): δ, 14.0 (CH3), 60.5 (CH2), 107.0,108.0, [Ar-C, 120.8, 122 - 129.63, 130.67, 131.01, 131.68, 137.47], 145.17, 152.0, 153.0, 154.0, 155.5, 158.3, 161.0, 163.5 (CO), 166.0 (CO); EIMS (m/z,%) 502 (M+, 20). Anal. calcd. for C29H22N6O3 (502.5); C, 69.31; H, 4.41; N, 16.72. Found: C, 69.24; H, 4.44; N, 16.49%.
= 3348, 3164 (NH2), 1671, 1634 (CO) cm−1; 1H NMR (CDCl3): δ,1.25 (t, 3H, CH3), 4.31 (q, J = 7.3, 2H, CH2), 6.75 (s, 2H. NH2, D2O exchangeable), 7.20 - 7.80 (m, 15H, Ar-H); 13C NMR (DMSO-d6, 75 MHz): δ, 14.0 (CH3), 60.5 (CH2), 107.0,108.0, [Ar-C, 120.8, 122 - 129.63, 130.67, 131.01, 131.68, 137.47], 145.17, 152.0, 153.0, 154.0, 155.5, 158.3, 161.0, 163.5 (CO), 166.0 (CO); EIMS (m/z,%) 502 (M+, 20). Anal. calcd. for C29H22N6O3 (502.5); C, 69.31; H, 4.41; N, 16.72. Found: C, 69.24; H, 4.44; N, 16.49%.
Reaction of 4 with potassium thiocyanate: Synthesis of 1,3,6-triph- enyl-9-thioxo-9,10-dihydropyrimido[4,5-b]pyrido[4,5-d][1,2,4]triazolo[4,3-a]pyrimidin-5,7(1H,8H)-dione (5).
To a solution of 4 (1 g, 1.99 mmol) and dioxane in acetic acid (20 ml) and drops 10% HCl, potassium thiocyanate (0.1 g, 1.03 mmol) was added and the mixture was refluxed for 7 h. After cooling, the solvent was evaporated and 20 ml H2O was added. The solid formed was filtered, washed with H2O and crystallized from ethanol and drops of dioxane to give 5 as pale yellow crystals.
Yield: 0.9 g (87.73%); m.p. 270˚C; IR (KBr):  = 3277, 3074 (2NH), 1672, 1630 (CO) cm−1; 1H NMR (DMSO-d6,): δ, 7.10 - 8.25 (m, 15H, Ar-H), 9.45 (s, 1H, NH), 11.19 (s, 1H, NH); 13C NMR (DMSO-d6, 75MHz): δ, 119.8, 113.0, 116.0, [Ar-C, 122 - 129.53, 130.0, 131.0, 132.8, 137.5], 140.99, 143.46, 146.46, 154.32, 155.45, 156.0, 161.8, 167.46; EIMS (m/z,%) 515 (M+, 10). Anal. calcd. for C28H17N7O2S (515.55); C, 65.23; H, 3.32; N, 19.02; S,6.22. Found: C, 65.00; H, 3.30; N, 18.70; S, 6.15%.
= 3277, 3074 (2NH), 1672, 1630 (CO) cm−1; 1H NMR (DMSO-d6,): δ, 7.10 - 8.25 (m, 15H, Ar-H), 9.45 (s, 1H, NH), 11.19 (s, 1H, NH); 13C NMR (DMSO-d6, 75MHz): δ, 119.8, 113.0, 116.0, [Ar-C, 122 - 129.53, 130.0, 131.0, 132.8, 137.5], 140.99, 143.46, 146.46, 154.32, 155.45, 156.0, 161.8, 167.46; EIMS (m/z,%) 515 (M+, 10). Anal. calcd. for C28H17N7O2S (515.55); C, 65.23; H, 3.32; N, 19.02; S,6.22. Found: C, 65.00; H, 3.30; N, 18.70; S, 6.15%.
Reaction of 5 with hydrazonoyl chlorides, (N-phenyl benzenecarbohydrazonoyl chloride(1a) & N-Phenyl-2-Phenylamino-2-oxoethanehydrazo- noyl chloride (1b)): Synthesis of 1,3,6,9,11-pentasubstituted-pyrido[3,2- f:6,5-f']bis([1,2,4]triazolo[4,3-a]-pyrimidin-5(1H)-ones (6).
General procedure:
Toa mixture of 1,3,6-triphenyl-9-thioxo-9,10-dihydropyrimido[4,5-b]pyrido [4,5-d][1,2,4]triazolo[4,3-a]pyrimidine-5,7(1H,8H)-dione (5) (0.5 g, 1 mmol) and the appropriate hydrazonoyl chloride 1a or 1b (1 mmol) in 15 ml dioxane and triethylamine (0.15 ml, 1 mmol) were added. The reaction mixture was heated under reflux until the evolution of hydrogen sulfide gas had ceased, for (24 h) (as evidenced by TLC). The reaction mixture, on cooling, afforded only one product in each case (TLC) and then the solvent was distilled off, and the residue was cooled. The solid formed was filtered, washed with H2O, and crystallized from ethanol: dioxane (v/v, 9:1) to give 6.
1,3,6,9,11-pentaphenylpyrido[3,2-f:6,5-f']bis([1,2,4]triazolo[4,3-a]-pyrimidin-5(1H)-ones (6a).
Yield: 0.2 g (30.52%);Yellow crystals; m.p. 294˚C; IR (KBr): 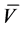 = 1671, 1636 (CO) cm−1; 1H NMR (DMSO-d6): δ, 7.06 - 8.26 (m, 25H, Ar-H); 13C NMR (DMSO-d6, 75MHz): δ, [Ar-C, 122-129.53, 130.0, 131.0, 132.8, 137.5, 139.0], 141.5, 142.0, 144.5, 148.8, 152.0, 154.2, 155.5, 159.0, 163.0; EIMS (m/z,%) 676 (M++1, 35). Anal. calcd. for C41H25N9O2(675.7); C, 72.88; H, 3.73; N, 18.66. Found: C, 72.50; H, 3.80; N, 18.50%.
= 1671, 1636 (CO) cm−1; 1H NMR (DMSO-d6): δ, 7.06 - 8.26 (m, 25H, Ar-H); 13C NMR (DMSO-d6, 75MHz): δ, [Ar-C, 122-129.53, 130.0, 131.0, 132.8, 137.5, 139.0], 141.5, 142.0, 144.5, 148.8, 152.0, 154.2, 155.5, 159.0, 163.0; EIMS (m/z,%) 676 (M++1, 35). Anal. calcd. for C41H25N9O2(675.7); C, 72.88; H, 3.73; N, 18.66. Found: C, 72.50; H, 3.80; N, 18.50%.
1,3,6,11-tetraphenyl-9-phenylaminocarbonylpyrido[3,2-f:6,5-f']di([1,2,4] triazolo[4,3-a]-pyrimidin-5(1H)-ones (6b).
Yield: 0.2 g (28.69%); Yellow solid; m.p. 272˚C - 273˚C; IR (KBr):  = 3433 (NH), 1684, 1605 (CO) cm−1; 1H NMR (DMSO-d6): δ, 7.17 - 8.23 (m, 25H, Ar-H), 10.77 (s, 1H, NH); 13C NMR (DMSO-d6, 75MHz): δ, [Ar-C, 122 - 129.5, 130.5, 131.8, 132.9, 137.5, 139.2], 141.0, 142.2, 144.3, 148.5, 151.3, 152.5, 154.2, 155.5, 158.5, 164.0; EIMS (m/z,%) 718 (M+, 25). Anal. calcd. for C42H26N10O3 (718.72); C, 70.19; H, 3.65; N, 19.49. Found: C, 70.0; H, 3.50; N, 19.30%.
= 3433 (NH), 1684, 1605 (CO) cm−1; 1H NMR (DMSO-d6): δ, 7.17 - 8.23 (m, 25H, Ar-H), 10.77 (s, 1H, NH); 13C NMR (DMSO-d6, 75MHz): δ, [Ar-C, 122 - 129.5, 130.5, 131.8, 132.9, 137.5, 139.2], 141.0, 142.2, 144.3, 148.5, 151.3, 152.5, 154.2, 155.5, 158.5, 164.0; EIMS (m/z,%) 718 (M+, 25). Anal. calcd. for C42H26N10O3 (718.72); C, 70.19; H, 3.65; N, 19.49. Found: C, 70.0; H, 3.50; N, 19.30%.
Reaction of hydrazonoyl chlorides (1a-d) with 4-amino-6-[(2-thiophen-2- yl)ethenyl]-3-thioxo-3,4-dihydro-[1,2,4]triazin-5(2H)-one (11).
General procedure:
To a mixture of 4-amino-6-[(2-thiophen-2-yl)ethenyl]-3-thioxo-3,4-dihydro- [1,2,4]triazin-5(2H)-one (11) (2 mmol) [33] and an appropriate hydrazonoyl chlorides (1a-d) (1 mmol) in chloroform (20 ml) was added triethylamine (0.4 ml, 2.5 mmol) and the reaction mixture was stirred at room temperature for (15 - 24 h). The solid that precipitated was filtered off, washed with water, dried and finally crystallized from ethanol to give chromatographically, impure products, which were purified by column chromatography (EA/CHCl3, 10:90) to give pure yellow crystals (16a-d).
16a: 1,3-diphenyl-7-[(E)-2-(thiophen-2-yl)ethenyl]-1,4,9,9a-tetrahydro- 6H-[1,2,4]triazino[4,3-b][1,2,4,5]tetrazin-6-one.
Yield: 0.2 g, 24.39%; yellow solid; m.p. 174˚C; IR (KBr)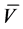 , cm?1 = 3432, 3269 (2NH), 1657 (CO); 1H NMR (CDCl3): δ 7.0 - 8.2 (m, 15 H, Ar-H & CH=CH, thienyl-3H), 8.8 (s, 1H, NH); 13C NMR δ: 119.4, 123.68, 125.93, 126.8, 127.0, 127.63, 127.99, 128.9, 129.16, 129.28, 129.8, 132.12, 139.66, 141.37, 142.15, 146.6, 147.0, 149.99, 164.0. MS, m/z (%): 412 (M+, 25). Anal. Calcd. for C22H16N6OS (412.47): C, 64.06; H, 3.91; N, 20.38; S, 7.77. Found: C, 63.92; H, 4.05; N, 20.10; S, 7.60%.
, cm?1 = 3432, 3269 (2NH), 1657 (CO); 1H NMR (CDCl3): δ 7.0 - 8.2 (m, 15 H, Ar-H & CH=CH, thienyl-3H), 8.8 (s, 1H, NH); 13C NMR δ: 119.4, 123.68, 125.93, 126.8, 127.0, 127.63, 127.99, 128.9, 129.16, 129.28, 129.8, 132.12, 139.66, 141.37, 142.15, 146.6, 147.0, 149.99, 164.0. MS, m/z (%): 412 (M+, 25). Anal. Calcd. for C22H16N6OS (412.47): C, 64.06; H, 3.91; N, 20.38; S, 7.77. Found: C, 63.92; H, 4.05; N, 20.10; S, 7.60%.
16b: 3-Phenylamido-1-phenyl-7-[(E)-2-(thiophen-2-yl)ethenyl]-1,4,9,9a- tetrahydro-6H-[1,2,4]triazino[4,3-b][1,2,4,5]tetrazin-6-one.
Yield: 0.2 g, 22. 15%; yellow solid; m.p. 211˚C - 214˚C; IR (KBr)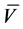 , cm?1 = 3381, 3160 (2NH), 1645, 1600 (2CO); 1H NMR (DMSO-d6): δ 7.01 - 7.76 (m, 15 H, Ar-H & CH=CH, thienyl-3H), 10.18 (s, 1H, NH), 11.7 (s, 1H, NH); 13C NMR δ:114.67, 115.85, 120.28, 120.97, 122.78, 122.98, 124.16, 127.28, 128.67, 129.35, 129.59, 138.29, 142.19, 163.0, 168.0 . MS, m/z (%): 455 (M+, 28). Anal. Calcd. for C23H17N7O2S (455.49); C, 60.65; H, 3.76; N, 21.53; S, 7.04. Found: C, 60.22; H, 3.50; N, 21.40; S, 7.00%.
, cm?1 = 3381, 3160 (2NH), 1645, 1600 (2CO); 1H NMR (DMSO-d6): δ 7.01 - 7.76 (m, 15 H, Ar-H & CH=CH, thienyl-3H), 10.18 (s, 1H, NH), 11.7 (s, 1H, NH); 13C NMR δ:114.67, 115.85, 120.28, 120.97, 122.78, 122.98, 124.16, 127.28, 128.67, 129.35, 129.59, 138.29, 142.19, 163.0, 168.0 . MS, m/z (%): 455 (M+, 28). Anal. Calcd. for C23H17N7O2S (455.49); C, 60.65; H, 3.76; N, 21.53; S, 7.04. Found: C, 60.22; H, 3.50; N, 21.40; S, 7.00%.
16c: 3-Acetyl-1-phenyl-7-[(E)-2-(thiophen-2-yl)ethenyl]-1,4,9,9a-tetra- hydro-6H-[1,2,4]triazino[4,3-b][1,2,4,5]tetrazin-6-one.
Yield: 0.1 g, 13.33%; pale yellow solid; m.p. 105˚C; IR (KBr)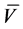 , cm?1 = 3350, 3190 (2NH), 1700, 1630 (2CO); 1H NMR (DMSO-d6): δ 2.49 (s, 3H, CH3), 6.9 - 8.08 (m, 10 H, Ar-H & CH=CH, thienyl-3H), 8.9 (s, 1H, NH); 13C NMR δ: 24.1 (CH3), 116.2, 119.38, 124.13, 126.98, 128.05, 128.55, 128.82, 129.19, 140.05, 143.09,162.0, 168 . MS, m/z (%): 378 (M+, 43). Anal. Calcd. for C18H14N6O2S (378.41), C, 57.13; H, 3.73; N, 22.21; S, 8.47. Found: C, 56.86; H, 4.00; N, 21.90; S, 8.30%.
, cm?1 = 3350, 3190 (2NH), 1700, 1630 (2CO); 1H NMR (DMSO-d6): δ 2.49 (s, 3H, CH3), 6.9 - 8.08 (m, 10 H, Ar-H & CH=CH, thienyl-3H), 8.9 (s, 1H, NH); 13C NMR δ: 24.1 (CH3), 116.2, 119.38, 124.13, 126.98, 128.05, 128.55, 128.82, 129.19, 140.05, 143.09,162.0, 168 . MS, m/z (%): 378 (M+, 43). Anal. Calcd. for C18H14N6O2S (378.41), C, 57.13; H, 3.73; N, 22.21; S, 8.47. Found: C, 56.86; H, 4.00; N, 21.90; S, 8.30%.
16d: Ethyloxo-1-phenyl-7-[(E)-2-(thiophen-2-yl)ethenyl]-1,6,9,9a-tetra- hydro-4H-[1,2,4]triazino[4,3-b][1,2,4,5]tetrazin-3-carboxylate.
Yield: 0.2 g, 24.69%; orange solid; m.p. 1118˚C; IR (KBr)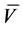 , cm?1 = 3431, 3000 (2NH), 1737, 1676 (2CO); 1H NMR (DMSO-d6): δ 1.2 (t, 3H, CH3), 4.18 (q, J = 7.3, 2H, CH2), 6.99 - 7.37 (m, 10 H, Ar-H & CH=CH, thienyl-3H), 10.79 (s, 1H, NH); 13C NMR δ: 14.0 (CH3), 61.5 (CH2), 114.5, 116.9, 119.38, 122.65, 126.98, 128.05, 129.3, 140.05, 142.63, 162.5, 169.5. MS, m/z (%): 408 (M+, 38). Anal. Calcd. for C19H16N6O3S (408.43); C, 55.87; H, 3.95; N, 20.58; S, 7.85. Found: C, 55.60; H, 4.20; N, 20.40; S, 8.00%.
, cm?1 = 3431, 3000 (2NH), 1737, 1676 (2CO); 1H NMR (DMSO-d6): δ 1.2 (t, 3H, CH3), 4.18 (q, J = 7.3, 2H, CH2), 6.99 - 7.37 (m, 10 H, Ar-H & CH=CH, thienyl-3H), 10.79 (s, 1H, NH); 13C NMR δ: 14.0 (CH3), 61.5 (CH2), 114.5, 116.9, 119.38, 122.65, 126.98, 128.05, 129.3, 140.05, 142.63, 162.5, 169.5. MS, m/z (%): 408 (M+, 38). Anal. Calcd. for C19H16N6O3S (408.43); C, 55.87; H, 3.95; N, 20.58; S, 7.85. Found: C, 55.60; H, 4.20; N, 20.40; S, 8.00%.
2.2. Antimicrobial Assay
Cultures of two bacterial species namely, Escherichia coli EC, and Staphylococcus aureus SA as well as well as two fungal species, namely Aspergillus flavus AF, and Candida albicans CA were used to investigate the antimicrobial activity of eight products, namely 4, 5, 6a, 6b and 16a-16d. The antimicrobial activity tests were carried out in the Microbiology Division of Microanalytical Center of Cairo university using the diffusion plate technique [34] [35] . The latter technique was carried out by pouring a spore suspension of the fungal species (1 cm3 of sterile water contains approximately 108 conidia) or spreading bacterial suspension over a solidified malt agar medium. The layer is allowed to set for 30 min. A solution of the test compounds (1.0 g = cm3) in DMSO was placed onto sterile 5mm filter paper discs and allowed to dry, then the discs were placed on the centre of the malt agar plate and incubated at optimum incubation temperature 28˚C ± 2˚C. The bactericide Ampicillinand, the fungicide Amphotericin B were used as standards under the same conditions. Measurements were considered after 72 h for fungi and 24 h for bacteria. The results are summarized in table.
3. Results and Discussion
3.1. Chemistry
The first required starting material in this part, 7-amino-1,3-diphenyl[1,2,4] triazolo[4,3-a]pyrimidin-5(1H)-one (3) [21] was prepared by reaction of the hydrazonoyl chloride 1a with 6-aminouracil-2-thione (2) [32] in dioxane and triethyamine. Reaction of the compound 3 with benzaldehyde and ethyl cyanoacetate in dimethylformamide afforded the corresponding 8-amino-7-ethoxycar- bonyl-1,3,6-triphenylpyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one
(4). The latter compound was cyclized by refluxing in a mixture of potassium thiocyanate and dioxane in the presence of concentrated hydrochloric acid affording1,3,6-triphenyl-9-thioxo-9,10-dihydropyrimido[4,5-b]-pyrido[4,5-d][1,2, 4]triazolo[4,3-a]pyrimidin-5,7(1H,8H)-dione (5) (Scheme 1).
The 1H NMR spectrum of each of the resulting products 4 and 5 is in agreement with the given structure [see experimental part]. Hence, the product 4 revealed characteristic signals for ethyl ester and amino groups at d = 1.25 (t, 3H, CH3), 4.31 (q, J = 7.3, 2H, CH2), 6.75 (s, 2H. NH2, D2O exchangeable), while the product 5 revealed characteristic signals of new 2 NH at d = 9.45 (s, 1H, NH, D2O exchangeable) and 11.19 (s, 1H, NH, D2O exchangeable). Reaction of
Scheme 1. Reaction of hydrazonoyl chloride with 1,3,6-triphenyl-9,10-dihydropyrimido [4,5-b]pyrido[4,5-d][1,2,4]triazolo[4,3-a]pyrimidin-5,7[1H,i8iH]-dione(5).
1,3,6-triphenyl-9-thioxo-9,10-dihydropyrimido[4,5-b]pyrido[4,5-d][1,2,4]triazolo[4,3-a]pyrimi-din-5,7(1H,8H)-dione (5) with hydrazonoyl chlorides 1a,b were carried out in dioxane in the presence of triethylamine at reflux until the evolution of hydrogen sulfide gas had ceased. The reaction mixture, on cooling, afforded only one product in each case (as evidenced by TLC). Both spectroscopic data and elemental analyses were consistent with structure 6 or 7 (Scheme 1). An immediate distinction between these two structures was reached by comparison of the 13C NMR spectra with those of similar annulated pyrimidinones. Literature report [36] has shown that the chemical shift for the carbonyl carbon in 4-pyrimidi- none derivatives is markedly affected by the nature of the adjacent nitrogen (N8) (pyrrole type in structure 6 and pyridine type as in structure 7). For example, the 13C NMR spectra of 6a taken as typical example of the series prepared, revealed the signals of the carbonyl carbon of the pyrimidinone ring residue at d = 163.15. Such chemical shift values are similar to those of annulated pyrimidines with N8 pyrrole type rather than those of N8 pyridine type (Chart 1).
The chemical shift values of the annelatedpyrimidinones A and B are shown in Chart 1 [21] [22] . Since the values found for the isolated products are similar to those of A and not B, the products were assigned structure 6.
On the basis of this similarity, the isolated products were assigned structure 6 and the isomeric structure 7 was excluded. As depicted in Scheme 1. The reaction proceeded through S-alkylation [37] to give S-alkylated products 8 followed by Smiles rearrangement [38] , afforded intermediate 9 which consumed insitu via elimination of hydrogen sulfide gas to give the desired products 6a,b (Scheme 2). The assignment for the structure products and reaction mechanism can be manifested by alternate synthesis. Thus, refluxing of 7-amino-1,3-di- phenyl[1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one (3) with benzaldehyde in acetic acid afforded directly the product 6a (Scheme 3) [22] .
Also the reaction of 6-aminouracil-2-thione (2) with benzaldehyde in acetic acid afforded the compound 10 [39] , which reacted with hydrazonoyl chloride (1a) to yield the product 6a [22] (Scheme 3). The product 6a obtained by the alternate synthesis found to be identical in all respects (mp, mixed mp, IR and 1HNMR) with the product produced from the reaction of 5 with 1a in Scheme 1.
Our study was extended to introduce 1,2,4-triazine containing thiophene moiety which has wide applications in different biological and medicinal fields beside its application in organic chemistry [40] [41] [42] [43] . Thus hydrazonoyl chlorides (1a-d) reacted with 4-amino-6-[(2-thiophen-2-yl)ethenyl]-3-thioxo-
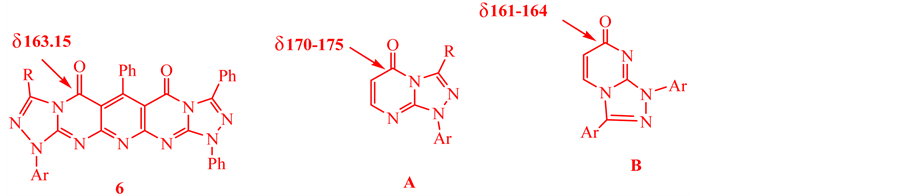
Chart 1. 13C NMR Shifts of strategic carbon atoms.

Scheme 2. Mechanism of the formation of 1,3,6,9,11,-pentasubstitited-pyrido [3,2-f:6,5-f] di([1,2,4])trizaolo[4,3-a]pyrimidin-5(1H)-one (6).

Scheme 3. Alternate Synthesis of 1,3,6,9,11-pentasubstituted-pyrido [3,2-f:6,5-f]di ([1,2,4])trizaolo[4,3-a]pyrimidin-5(1H)-one (6).
3,4- dihydro-[1,2,4]triazin-5(2H)-one (11) [40] in chloroform and triethylamine at room temperature for 15 - 24 h (according to TLC, no start was found) to give chromatographically impure product. The latter was purified by column chromatography by using eluent of a mixture of ethyl acetate/CHCl3 (10: 90) to give one single pure product. The structure of the product obtained may be 13 or 16 (Scheme 4).
It was initially anticipated that such reactions would yield the respective 1,2,4- triazino[2,1-b][1,3,4,5]thiatriazine (13) by analogy to the reactions of 1a-d with 2-aminothiophenol, which were reported to afford benzothiadiazine derivatives [22] [39] . Unexpectedly, the products isolated from the reactions of 1a-d with 11

Scheme 4. reaction of 4-amino-6-[2-(thiophen-2-yl) ethenyl]-3-thioxo-3,4-dihydro- 1,2,4-triazin-5(2H)-one(11) with Hydrazonoyl Chlorides 1a-d.
were identified as 1,3-disubstituted-7-[(E)-2-(thiophen-2-yl)ethenyl]-1,4,9,9a- tetrahydro-6H-[1,2,4]triazino[4,3-b][1,2,4,5]-tetrazin-6-one (16) and not 13. The structures of the latter products were elucidated on the basis of their spectra (1H & 13C NMR, IR, and MS) and microanalyses. For example, the 1HNMR spectra of the product 16a showed NH proton signal at d 8.8, and it was lacking the N-NH2 signal at d 6.5, which is characteristic of the starting substrate 11. The mass spectra of 16a were also consistent with its assigned structures, especially its molecular ion peak. The formation of 16a-d from 1a-d and 11 can be rationalized in terms of the pathway outlined in Scheme 4. It is suggested that the reaction begins with the formation of the respective hydrazide 14, that cyclized to 15, which in turn give 16 with concurrent elimination of hydrogen sulfide (Scheme 4) [41] [42] .
3.2. Antimicrobial Activity
The test results revealed that all compounds exhibited moderate activity against the two bacterial species except, 5, 6b and all compounds showed no activity against all fungal species, except compounds 5, and 16c against Candida albicans (CA) (Table 1).
![]()
Table 1. Antibacterial and antifungal activities of some of the synthesized compounds.
*IZD = 2 - 10 mm beyond control = + (low activity). IZD = 11 - 24 mm beyond control = ++ (moderate activity). IZD = 25 - 35 mm beyond control = +++ (high activity).
4. Conclusion
In this report, it is found that hydrazonoyl chlorides (1) are good precursor to synthesize fused heterocycles. Thus the reaction of such hydrazonoyl chlorides (1) with 1,3,6-triphenyl-9-thioxo-9,10-dihydropyrimido[4,5-b]pyrido[4,5-d][1,2,4]triazolo[4,3-a]pyrimidin-5,7(1H,8H)-dione (5) and 4-amino-6-[(2-thiophen-2-yl) ethenyl]-3-thioxo-3,4-dihydro-[1,2,4]triazin-5(2H)-one (11) afforded to new functionalized fused heterocycles. Spectral data revealed that such fused heterocycles have the structures of 1,3,6,9,11-penta-substitutedpyrido[3,2-f:6,5-f']bis- ([1,2,4]triazolo[4,3-a]-pyrimidin-5(1H)-ones (6) and 1,3-disubstituted-7-[(E)-2- (thiophen-2-yl)ethenyl]-1,4,9,9a-tetrahydro-6H-[1,2,4]triazino[4,3-b][1,2,4,5]- tetrazin-6-ones (16) respectively. The antibacterial and antifungal activity screening of the prepared compounds revealed moderate activity against the bacterial species.
Acknowledgements
The authors thank King Abdulaziz City for Science and Technology (KACST), Kingdom of Saudi Arabia (KSA) for the financial support under project number AT36-062. This paper is dedicated to the memory of Prof. Dr. Dr. Ahmad Sami Abdelshakour Shawali, Cairo University, Egypt.