1. Introduction
Electrochromism is a topic that has attracted a great deal of interest from researchers because of its potential application in various areas, such as photonics, optics, electronics, architecture, etc. Electrochromic (EC) properties can be found in almost all the transition-metal oxides and their properties have been investigated extensively in the last decades [1] . These oxide films can be coloured anodically (Ir, Ni) or cathodically (W, Mo); however, WO3 is clearly the preferred material for applications. This is principally due to the fact that WO3-based electrochromic devices (ECD) have normally a faster response time to a change in voltage and a larger coloration efficiency (CE) as compared to devices based on other electrochromic materials. Recently Gran-qvist et al. [2] have made a comprehensive review of nanomaterials for benign indoor environments. In this report, the authors show the characteristic data for a 5 ´ 5 cm2 flexible EC foil incorporating WO3, and NiO modified by the addition of a wide bandgap oxide such as MgO or Al2O3, PMMA-based electrolyte, and ITO films. Durability of the EC devices was demonstrated in performing several tens of thousands of coloration/ bleaching cycles, and the device optical properties were found to be unchanged for many hours. Using solid-state electrolytes like lithium-ion conducting materials, one can make all-solid-state windows. Among numerous Li conductors, perovskite-type oxides (ABO3) show promising lithium high ion conductivity at room temperature.
Recently, many works [3] [4] [5] [6] have shown that the new family of perovskite structure La0.67−xLi3xTiO3 materials (hereafter called as LLTO) give a large Li+ ionic conductivity. At room temperature, the conductivity of LLTO films possesses a value up to 10−5 S/cm. Recently, Li et al. [7] reported the preparation of LLTO thin films by electron beam deposition, and their results showed that amorphous LLTO thin films deposited by e-beam with ionic conductivity of 10−5 S/cm that is suitable for application as a large-area electrolyte for thin-film batteries and electrochromic windows. We have shown that at room temperature LLTO bulk materials prepared by conventional solid-state reaction possess a larger Li+ ion conductivity from 10−4 ÷ 10−3 S/cm [8] .
In this work, the e-beam evaporation method was also used for deposition of LLTO thin films. The solid-state electrolyte LLTO films were used in WO3-based ECD devices working as all-solid-state electrochromic mirror mirrors. The performance of the electrochromic mirrors was also presented.
2. Experiment
The La0.67−xLi3xTiO3 crystalline powder was synthesized by ball grinding method according to the following reaction:
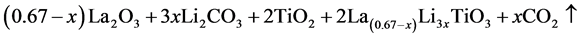 (1)
(1)
where the values of x was chosen as 0.11, with this value the ionic conductivity was the best one as reported in [8] . These samples were made by following procedures: Appropriate stoichiometric amount of dehydrated La2O3 (99.9%), Li2CO3 (99.9%) and TiO2 (99.9%) were purchased from Aldrich Chemicals Ltd. The mixture was dried and annealed at 800oC in air atmosphere for 4h in order to eliminate CO2. Afterwards, the mixtures were ball-grinded by a “Fritsch Mill Model Pulverisette-6” machine, for a time duration ranging from 4 h to 6 h and 8 h. LLTO powder then was pressed by using “3636-X press” (35 ton Lab. Pellets Press) to get targets of a size of 8 mm in diameter and 5 mm in thickness. By using e-beam evaporation, LLTO films were deposited from these targets. The base pressure of the evaporation chamber was 1.5 × 10−4 Pa. Oxygen gas flux was regulated to maintain the chamber pressure at about 5.5 × 10−2 Pa. Corning glasses coated by ITO (Indium Tin Oxide) were used as substrates, the temperature of the substrates was kept at 450˚C. The substrate-target distance was about 25 cm. The deposition was maintained by use of a vibrating quartz crystal microbalance. 300 nm- thick LLTO films used for electrochromic display were deposited onto previously deposited WO3/ITO (namely LLTO/WO3/ITO). 350 nm-thick WO3 films coated on ITO substrates (WO3) were prepared also by e-beam deposition according to the procedure which was described in Ref. [9] . LiMn2O4 served as a Li+ ion resource (or cathode in all-solid-state battery) was e-beam deposited onto LLTO/WO3/ITO, then Al coating onto the LiMn2O4 layer was metallized successively by thermal vacuum evaporation in the same chamber. Thus ECD devices working as a smart reflective mirror was prepared in a laminar structure of Al/LiMn2O4/LLTO/WO3/ITO (further abbreviated to “LWM” mirror). For characterization of transmittance, LTTO films were e-beam deposited on glass substrates. For conductivity measurements, an Al/LLTO/ITO sandwich structure was made by vacuum evaporation of Al coatings on the surface of LLTO/ITO samples with a working electrode of 5 ´ 5 mm2.
The structure of LLTO films was characterized by X-ray diffraction (XRD) patterns recorded by a Siemens D-5000 X-ray diffractometer with Cu-Ka radiation. The morphology and cross-section of the LLTO thin films were investigated by field emission scanning electron microscopy (FE-SEM). The ionic conductivity of thin films was characterized on Auto. Lab Potentiostat-PGS30 with a FRA-2 impedance spectra technique. Optical measurement was carried-out on a Jasco V-570 photospectrometer.
3. Results and Discussion
3.1. Crystalline Structure, Morphology and Ionic Conductivity of the Samples
Figure 1 shows X-ray diffraction patterns of a deposited film. XRD patterns demonstrated that the LLTO films were crystallized with submicrostructured grains. From the XRD peaks one can determine grain size (t) using the Scherrer formula:
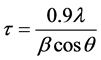 (2)
(2)
where l is wavelength of the X-ray used, b the peak width of half height in radians and q the Bragg angle of the considered diffraction peak [10] . The calculation result showed that the average size of the crystalline grains in the LLTO film is ~100 nm.
Figure 2 shows SEM images of surface and cross-section of the LLTO thin film (x = 0.11). It is seen that, the as-deposited films have a very dense surface with grain size of ca. 100 nm (Figure 2(a)) and thickness of the film is about 300 nm (Figure 2(b)). The film has a good adherence with the substrate.
From impedance spectra measurements, the ionic conductivity of the ceramic LLTO targets was found to be of 3.25 ´ 10−3 S/cm that consists with the value reported in [8] .
![]()
Figure 1. XRD patterns of a LLTO film sample with x = 0.11.
![]()
Figure 2. FE-SEM image of LLTO thin film (a) and Cross-section of SEM image of a LLTO thin film (b). The film thickness d = 300 nm.
Figure 3(a) shows the complex impedance plot (Z’ versus Z” plot) measured in the frequency range 100 Hz to 1.0 MHz at room temperature for a LLTO film. The impedance plot consists of two semicircles, respectively characterizing by a Li+ ion conducting process in electrolyte and a process at interface of electrolyte and electrodes. The resistance of thin films was determined by using a software program for fitting experimental data and theoretical spectrum of equivalent circuit available. The proposed equivalent circuit schema is shown in Figure 3(b). In part (I), R1 marks serial resistance of circuit. In part (II), R2 and C1 are the resistance of electrolyte and capacitance of the passive film of the electrolyte surface, respectively. In part (III), R3 and C2 are the resistance and capacitance of the passive film on the electrodes, respectively; R4 and C3 are resistance and double-layer capacitance of electrolyte/electrode interfaces. The ionic conductivity (s) can be calculated from the value of electrolyte resistance R2 according to the formula:
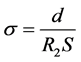 (3)
(3)
where d and S are the thickness (300 nm) and electrode area (0.25 cm2) on the LLTO thin film, respectively.
![]()
![]() (a) (b)
(a) (b)
Figure 3. Cole-Cole plots of the impedance of LLTO with Li contents x = 0.11 (a) and the equivalent circuit schema (b).
From the equivalent circuit schema fitting with the experimental curve, R2 was found to be of 2.18 W. In this case, the ionic conductivity of the films determined from formula (3) is as large as s = 5.50 ´ 10−5 S/cm, that is comparative with the conductivity of the LLTO thin films prepared by the pulsed laser deposition [11] . In comparison with bulk conductivity of the target material, the ionic conductivity of thin films is lower in two orders by magnitude. Such ion conducting decreased of thin film could be originated from i) decomposition of a part of lithium amount during evaporation process caused a lack of Li in LLTO thin films, ii) the presence of gain boundaries, and iii) an interface enhancement effect.
3.2. Electrochromic Properties
As-deposited LLTO thin films have slightly yellow color in visible range. Figure 4 shows transmittance spectrum of the film in wavelength range from 350 nm to 850 nm. The transmittance spectra of thin films is high in visible range, it is of 80% to 95%. This shows that the films prepared by electron beam deposition from the LLTO targets exhibit both the high Li-ionic conductivity and the high transmittance in visible wavelengths range.
For the LWM mirror, the in-situ reflectance spectra, obtained during coloration and bleaching at a polarized potential of −1.5 V and +1.5 V, respective to the ITO electrode, are given in Figure 5. From this figure one can see a large difference in reflectance spectra in the visible range of the device between the ex-situ (curve 1) and colored states corresponding to the applied voltage of −1.5 V to ITO (curve 2). With a polarization of +1.5 V applied to ITO, the reflectance spectrum was restored almost to the ex-situ state (curve 3). Note that, here reflectance spectra obtained from total spectrum that consists of i) transmittance through ITO/glass, WO3, LLTO and LiMn2O4 layers, then ii) reflectance from Al mirror, and finally transmittance through above four mentioned layers. Since ITO, LLTO and LiMn2O4 layers were not coloured under the electrochromic
![]()
Figure 4. Transmittance spectra of a LLTO (x = 0.11) thin film.
![]()
Figure 5. Electrochromic performance of the LWM device of a laminar structure of ITO/WO3/LLTO/LiMn2O4/Al as shown in the insertion.
performance, the reflectance of the LWM mirror is determined mainly by the coloration (or absorption) of the WO3 electrochromic layer.
The electrochromic performance of the WO3 electrode in LiClO3 + PC was described by cathodic reactions [12] [13] :
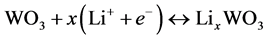 (4)
(4)
Thus in the LWM device the coloration/bleaching can be attributed to the insertion/extraction of Li+ ions in/out WO3 layer from LiMn2O4 through the solid LLTO electrolyte. The fact that the reflectance of the LWM mirror can be controlled by applying a polarized voltage onto ITO electrode proves that all-solid-state electrochromic devices can be utilized for fabrication of auto rear-view mirrors. Using these mirrors, the drives can prevent accidents which may be caused by radiating from the headlamp of the behind cars.
4. Conclusion
By using thermally ball-grinding method, submicro-structured La0.67−xLi3xTiO3 (with x = 0.11) powder was synthesized for the targets in e-beam deposition of the LLTO films. The best ionic conductivity of the films possesses a value as high as 5.50 ´ 10−5 S/cm. All-solid-state electrochromic devices of a laminar structure of Al/LiMn2O4/LLTO/ WO3/ITO were prepared. The reversible reflectance of the device was well controlled by polarization of potentials applied onto the ITO electrode. The obtained results suggest useful applications for electrochromic windows working as a smart reflectance mirror that can be used for auto rear-view mirrors.
Acknowledgements
This research was funded by the operational fund for science and technology of Hanoi Pedagogical University 2, under grant number C2015-18-05.