Spectrofluorometric Study of the Inclusion Complexation of Fluorescent Whitening Agents and β-Cyclodextrins ()
1. Introduction
Cyclodextrins are toroid-shaped, water soluble cyclic oligosacharides, mostly consisting of six, seven and eight D-glucopyranose units (α-CD, β-CD and γ-CD) linked by α-1,4 linkages. They are also known as cycloamyloses, cyclomaltoses and Schardinger dextrins. They are produced as a result of intramolecular transglycosylation reaction from degradation of starch by cyclodextrin glucanotransferase enzyme [1] .
Since cyclodextrins have a hydrophobic cavity of different dimensions depending on the number of glucose units forming the ring (α-CD: 5.2Å, β-CD: 6.6Å and γ-CD: 8.4Å), they can bind with various guest molecules forming supramolecular host- guest complexes [1] [2] [3] [4] .
The CD exterior area has 6, 7 or 8 hydroxyl groups and is fairly polar, whereas the interior of the cavity is relatively nonpolar. Therefore, these compounds have been studied as “hosts” for “guest” molecules capable of entering (completely or partially) in the cavity and forming noncovalent inclusion or occlusion complexes [5] [6] [7] . The non- bonding electron pairs of the glycosidic oxygen bridges are directed towards the inside of the cavity, which produce a high electron density and lend to the cavity some Lewis- base character [6] .
The inclusion of organic and organometallic compounds within the CD hydrophobic cavity and its effect on the photophysical properties of these molecules have been the subject of many investigations [5] [7] [8] [9] . These properties include fluorescence enhancement, intramolecular excimer/exciplex formation, twisted intramolecular charge transfer phenomena, fluorescence quenching phenomena and excited state in- tramolecular proton transfer reactions [10] [11] [12] [13] . The restrictive shape and size of the CDs also prompt changes in the excited state geometry. The complexed molecules can be included wholly or partially in the CD cavity, and many studies have focused on the ability of CDs to include guests of varying size in different stoichiometric ratios. Depending on the size of the host CD (α-, β- or γ-CD) and the size of the guest molecule, different guests/host stoichiometries are possible. A correct evaluation of the stoichiometry of the complex is crucial for the accurate determination of the formation constant, and an examination of this value can provide a clearer understanding of the factors affecting complexation [13] [14] .
It has been well established that a wide range of organic molecules in aqueous solution exhibit enhanced fluorescence when incorporated into CD hosts [1] [15] [16] . There are a number of reasons for this, including protection from fluorescence quen- chers (e.g. oxygen), the loss of rotational freedom (which can result in less efficient nonradiative decay and hence greater fluorescence), and the relatively nonpolar environment provided to the probe molecule by inclusion within the CD cavity. The latter is significant because many fluorescent probe molecules show significantly greater fluorescence in nonpolar than in polar environments. This fluorescence enhancement by cyclodextrin complexation has been exploited to increase the sensitivity of fluorescence-based trace detection techniques, such as the fluorescence detection of drugs and polychlorinated biphenyls [17] [18] [19] .
In the last few years, our research group has been interested in examining the formation and stability of inclusion complexes between fluorescent whitening agents (FWAs) and β-cyclodextrins, especially with modified CDs capable of binding to polymeric substrates, like cellulose [20] [21] . FWAs are very important chemical compounds used in the papermaking industry because they improve the optical properties of cellulose pulp. The FWAs are dyes that absorb light in the UV and violet region (usually 340 - 370 nm) of the electromagnetic spectrum and re?emit light in the blue region (typically 420 - 470 nm), causing a “whitening” effect, making materials look less yellow by increasing the overall amount of blue light reflected. The enhancement of fluorescence emission upon the formation of an inclusion complex was verified in a recent work [22] and now it is of interest to know the thermodynamic parameters involved in this complexation. These parameters reflect the stability and the concentration of the formed complexes and are useful to predict their facility to be formed spontaneously. The aim of this work is to determine thermodynamic parameters, such as the stability constant (Ks), 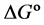 ,
, 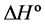 and
and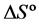 , for the formation of inclusion complexes between native β-cyclodextrin (βCD) and β-cyclodextrin monochlorotriazinyl (βCD-MCT) and the fluorophore 2,5-Bis(5-tert-butyl-benzoxazol-2yl)-thiophene as analytical reagent (UVOB) and commercial product (UVBNB) content 13%, v/v. The importance of UVOB is because this FWA is used as a commercial product (UVBNB) in papermaking industry to improve the optical properties of paper.
, for the formation of inclusion complexes between native β-cyclodextrin (βCD) and β-cyclodextrin monochlorotriazinyl (βCD-MCT) and the fluorophore 2,5-Bis(5-tert-butyl-benzoxazol-2yl)-thiophene as analytical reagent (UVOB) and commercial product (UVBNB) content 13%, v/v. The importance of UVOB is because this FWA is used as a commercial product (UVBNB) in papermaking industry to improve the optical properties of paper.
2. Materials and Methods
2.1. Materials
β-cyclodextrin native (βCD) and β-cyclodextrin-monochlorotriazinyl sodium salt (βCD-MCT) were kindly provided by Wacker Mexicana S.A. de C.V. (Mexico City, Mexico).
1,8-anilino-naphthalene sulfonic acid (1,8-ANS), 99.5%, were purchased from Aldrich (Milwaukee, WI, USA).
2,5-Bis(5-tert-butyl-benzoxazol-2yl)-thiophene, 99% analytical grade (UVOB) was purchased from Aldrich (Milwaukee, USA).
Uvitex BNB®, commercial optical brightener 13% (v/v) of 2,5-Bis(5-tert-butyl-ben- zoxazol-2yl)-thiophene (UVBNB), were kindly provided by Especialidades Químicas México, S.A, de C.V. (Mexico City, Mexico).
Di-sodium hydrogenphosphate, 99%, and sodium carbonate, 99.5%, were purchased from Aldrich (
Milwaukee
,
WI
,
USA
).
Other reagents were analytical grade and were used without further purification (Figure 1).
2.2. Methods
Fluorescence spectra were obtained in an Ocean Optics USB 2000 spectrofluorometer equipped with a continuous flow cell and pulsed xenon source light (PX-2). Temperature was controlled at 25˚C ± 0.1˚C with a Cole Parmer Polystat model 12112-00 to
![]()
Figure 1. 2,5-Bis(5-tert-butyl-benzoxazol-2yl)-thiophene.
carry out spectroscopic measurements. Carbonate buffer solution (0.1 mol∙dm−3) was used to control the pH at 10.5.
Formation of Inclusion Complexes
Fresh solutions were used in all measurements. An appropriate amount of the fluorophore solution was used in order to keep the same final concentration for all samples. The complexes were obtained by mixing a fixed concentration of fluorophore with different concentrations of cyclodextrin under magnetic stirring for 20 minutes and light protection. The flask was kept in a water bath to ensure constant temperature at 25˚C ± 0.1˚C. The reaction flask was connected to an external plastic tube to circulate the complex solution through a continuous flow cell, and then the fluorescence intensity was measured.
Since the UVOB is insoluble in water, inclusion complexes were prepared in acetone/water 90:10 (v/v) solutions for all the thermodynamic determinations in the system CD-UVOB.
Determination of Inclusion Complex Stoichiometry
The continuous variation plot method (Job Plot) was used to determinate the molar stoichiometry of the complexes formed. The difference in fluorescence intensity 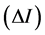 between the inclusion complex and the fluorophore alone solution, at the same concentration of the fluorescent whitening agent, was plotted as a function of the CD molar fraction
between the inclusion complex and the fluorophore alone solution, at the same concentration of the fluorescent whitening agent, was plotted as a function of the CD molar fraction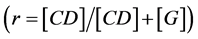 , where
, where  is the concentration of the guest molecule (fluorophore).
is the concentration of the guest molecule (fluorophore).
Determination of the Molar Constant of Complex Formation
Complex formation constants were determined using the proposed method by Benesi-Hildebrand [23] [24] [25] . Since the fluorescent intensity is directly proportional to the concentration of FWA, in this method a simple mass balance is used to determine the molar complexation constant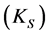 , based upon fluorescence intensity measurements under systematic variation of the cyclodextrin concentration. For conditions where the concentration of cyclodextrins is much greater than the equilibrium concentration of the complex, the Benesi-Hildebrand relationship for 1:1 complexes is given as:
, based upon fluorescence intensity measurements under systematic variation of the cyclodextrin concentration. For conditions where the concentration of cyclodextrins is much greater than the equilibrium concentration of the complex, the Benesi-Hildebrand relationship for 1:1 complexes is given as:
![]() (1)
(1)
where ![]() and
and ![]() are the intensity of fluorescence at the same concentration of the fluorophore alone and in the presence of known concentrations of cyclodextrin respectively.
are the intensity of fluorescence at the same concentration of the fluorophore alone and in the presence of known concentrations of cyclodextrin respectively. ![]() is the theoretical fluorescence intensity when all molecules of the fluorophore are complexed. The value of
is the theoretical fluorescence intensity when all molecules of the fluorophore are complexed. The value of ![]() was obtained from the intercept of the line resulting from plotting
was obtained from the intercept of the line resulting from plotting ![]() vs
vs ![]() and
and ![]() from the slope of the same plot respectively.
from the slope of the same plot respectively.
The thermodynamic parameters, Gibbs free energy change![]() , enthalpy change
, enthalpy change ![]() and entropy change
and entropy change ![]() for inclusion complexes were obtained from the Van’t Hoff Equation (2):
for inclusion complexes were obtained from the Van’t Hoff Equation (2):
![]() (2)
(2)
![]() and
and ![]() of the complex formation were calculated from the slope and intercept of the line resulting from plotting
of the complex formation were calculated from the slope and intercept of the line resulting from plotting ![]() versus 1/T.
versus 1/T.
![]() was calculated from Equation (3) that shows the relationship between the molar complexation constant
was calculated from Equation (3) that shows the relationship between the molar complexation constant![]() , the temperature (T) and the constant of ideal gases (R):
, the temperature (T) and the constant of ideal gases (R):
![]() (3)
(3)
3. Results and Discussion
Complexation of 1,8-ANS with βCD
The complex formation between 1,8-ANS and βCD was determined using the same experimental procedure for UVOB and UVBNB optical brighteners (except pH = 7.2), the value obtained was used as a reference to validate our experimental methods used here, since its thermodynamic parameters have been reported elsewhere [3] [26] .
Figure 2 shows the increased florescence as a function of the concentration of CD at a fixed concentration of 1,8-ANS (Job plot). The maximum of the curve at (r) between 0.5 and 0.6 indicated a stoichiometry of 1:1 for this complex, which is in agreement with data reported by other authors [3] [24] [27] .
Figure 3 shows the reciprocal of changes on the intensity of fluorescence as a function of the reciprocal of the concentration of BCD. The constant of complex formation
, calculated by using the Benesi-Hildebrand equation, was 113.09 ± 3 M−1.
In Figure 3, the concentration of 1,8-ANS was 0.01 mmol∙dm−
3 in
aqueous solution containing varying amounts (0.01 - 1.00 mmol∙dm−3) of βCD, λ = 513 nm.
The constant of complex formation between 1,8-ANS (and some derivatives) and βCD has been reported by other authors, those are summarized in Table 1. These data are in agreement with the constants calculated in this work and are similar to data reported for 8-anilino-1-naphthalene sulfonic acid (1,8-ANS) [26] and naphthalene sulfonate derivatives [3] . Even though there are variations in the numerical values, these belong to the same order of magnitude. These variations may be caused by the specific conditions used for each determination such as temperature, pH, range of concentrations, as well as the method employed for the determination of the constants [28] . The
![]()
Figure 2. Continuous variation plot of the complexation between 1,8-ANS and βCD.
![]()
Figure 3. Plot of the reciprocal of changes on the fluorescence intensity as a function of the reciprocal of the CD concentration for the 1,8-ANS:βCD inclusion complex.
![]()
Table 1. Equilibrium constants for [1,8-ANS]-βCD and 8-anilino-1-naphthalenesulfonate complexes.
*Fl = Fluorescence, NMR = Nuclear Magnetic Resonance.
value of constant complex formation between 1,8-ANS and βCD in this work is very close to reported in literature [26] , the slight difference can be attribute to the variation in pH value.
Complexation of UVOB with βCD and βCD-MCT
In order to evaluate the constant of inclusion complex, a system acetone/water (90/ 10, v/v) was used. CDs are soluble in water and UVOB is soluble in acetone. Hence, with this particular acetone/water ratio is possible to maintain both compounds in solution and therefore in contact to allow the complexation process. A sample used as blank probe was made with a mix of acetone/water (90/10, v/v) and βCD, and no fluorescence was observed.
UVOB alone (5.0 mmol∙dm−3) showed one maximum at 438 nm in its fluorescence intensity. The addition of different concentrations of βCD (0 - 40 mmol∙dm−3) showed also increased fluorescence intensity as shown in Figure 4. In this case, the solutions did not show a wavelength shift for the maximum fluorescence intensity. The increased fluorescence intensity with addition of incremental concentration of βCD, however, indicated the formation of an inclusion complex.
The thermodynamic parameters obtained for the inclusion complex constant for UVOB and βCD were ![]() = 4916 ± 137 M−1 and
= 4916 ± 137 M−1 and ![]() = −21.06 kJ∙mol−1. These values indicated an excellent formation of a stable complex, spontaneous process at 25˚C and pH = 10.5.
= −21.06 kJ∙mol−1. These values indicated an excellent formation of a stable complex, spontaneous process at 25˚C and pH = 10.5.
The fluorescence spectra of UVOB samples employing different concentrations of βCD-MCT are shown in Figure 5. The increased fluorescence intensity of the UVOB-
![]()
Figure 4. Fluorescence spectra of UVOB 5 mmol∙dm−3 upon addition of βCD (0) 0.0, (1) 5.0, (2) 10.0, (3) 20.0, (4) 30.0, (5) 40.0, mmol∙dm−3.
![]()
Figure 5. Fluorescence spectra of UVOB 0.01 mmol∙dm−3 upon addition of βCD-MCT (0) 0.0, (1) 0.1, (2) 0.2, (3) 0.3, (4) 0.4, (5) 1.0, mmol∙dm−3.
βCD-MCT complex with reference to the value of the compound alone indicates the formation of inclusion complexes. UVOB alone (0.01 mmol∙dm−3) showed two maxima on its fluorescence intensity at 438 and 485 nm. Addition of βCD-MCT (0.0 - 1.0 mmol∙dm−3) showed an increase in fluorescence intensity with no wavelength shifting for the maximum fluorescence intensity.
The thermodynamic parameters obtained were KS = 655 ± 19 M−1 and ![]() = −16.07 kJ∙mol−1. These values indicated the formation of a stable complex in a spontaneous process at 25˚C and pH = 10.5. Even though the value of KS seems to be low a 1:1, the stoichiometry indicated that a sufficient amount of complex was formed. This results show the possibility to improve the fluorescent effect and the water solubility of UVOB by means of a complex with βCD and βCD-MCT. Complexation could be considered as an alternative to solubilization in water of compounds poor soluble, it avoids the cost and difficulties otherwise involved in the use of organic solvents in the process.
= −16.07 kJ∙mol−1. These values indicated the formation of a stable complex in a spontaneous process at 25˚C and pH = 10.5. Even though the value of KS seems to be low a 1:1, the stoichiometry indicated that a sufficient amount of complex was formed. This results show the possibility to improve the fluorescent effect and the water solubility of UVOB by means of a complex with βCD and βCD-MCT. Complexation could be considered as an alternative to solubilization in water of compounds poor soluble, it avoids the cost and difficulties otherwise involved in the use of organic solvents in the process.
Complexation of UVBNB with βCD and βCD-MCT
Figure 6 and Figure 7 show the fluorescence spectra for the complexation between UVBNB-βCD, and UVBNB-βCD-MCT respectively. For UVBNB the fluorescence intensity spectrum was bimodal with maxima at 448 and 493 nm, being the latter the maximum for the fluorophore alone (line 0). For all samples with cyclodextrin (both βCD and βCD-MCT) the fluorescence intensity was higher, as compared to UVBNB alone. The fluorescence improvement in this solutions is attributed to the formation of the inclusion complex with cyclodextrins.
For complexation of UVBNB and βCD (lines 1-7 in Figure 6) the maxima were moved about 2 nm and 6 nm with respect the maxima observed in UVBNB alone. These shifts can be attributed to the changes in the conformation of the UVBNB mole-
![]()
Figure 6. Fluorescence spectra of UVBNB 0.5 mmol∙dm−3 upon addition of βCD (0) 0.0, (1) 1.0, (2) 2.0, (3) 3.0, (4) 4.0, (5) 6.0, (6) 7.0, (7) 8.0 mmol∙dm−3.
![]()
Figure 7. Fluorescence spectra of UVBNB 0.5 mmol∙dm−3 upon addition of βCD-MCT (0) 0.0, (1) 1.0, (2) 3.0, (3) 4.0, (4) 5.0, (5) 7.0, (6) 8.0, (7) 9.0, (8) 10 mmol∙dm−3.
cule when is in the cyclodextrin cavity. It is well known that inclusion phenomena cause changes in the maximum fluorescence intensity spectra of the guest molecules [1] [14] .
Complexation of UVBNB and βCD-MCT showed also shifts in their maxima, but these were higher than those observed in the UVBNB-βCD complex. The initial maximum of UVBNB alone at 493 nm was shifted at new maximum located at 443 nm, as a result of complexation process and the effect of the cavity of cyclodextrin (more hydrophobic ambient). Samples with increasing βCD-MCT concentration showed higher fluorescence intensity at the fixed UVBNB concentration (0.5 mmol∙dm−3).
Data results are treated according to the Benesi-Hildebrand equation, and these are shown in Figure 8. The complex associations constant ![]() for this system were 2552 ± 115 M−1 for native βCD and 1787 ± 75 M−1 for βCD-MCT. The Gibbs free energy
was calculated using the
value and results were
for this system were 2552 ± 115 M−1 for native βCD and 1787 ± 75 M−1 for βCD-MCT. The Gibbs free energy
was calculated using the
value and results were ![]() = −19.44 kJ∙mol−1 for βCD and
= −19.44 kJ∙mol−1 for βCD and ![]() = −18.55 kJ∙mol−1 for βCD-MCT. The higher
= −18.55 kJ∙mol−1 for βCD-MCT. The higher ![]() value for β-CD indicates a more stable complex formation. Modified cyclodextrins form inclusion complexes with lower
value for β-CD indicates a more stable complex formation. Modified cyclodextrins form inclusion complexes with lower ![]() values than their corresponding native CD, because the substituing groups cause steric hindrance in the complexation process [3] [14] .
values than their corresponding native CD, because the substituing groups cause steric hindrance in the complexation process [3] [14] .
The value of ![]() was calculated at 298, 303 and 308 ˚K for the estimation of
was calculated at 298, 303 and 308 ˚K for the estimation of ![]() and
and ![]() values. Thus,
values. Thus, ![]() for complexation of UVBNB-βCD-MCT was
for complexation of UVBNB-βCD-MCT was ![]() = −16.56 kJ∙mol−1 and for UVBNB-βCD was
= −16.56 kJ∙mol−1 and for UVBNB-βCD was ![]() = −11.35 kJ∙mol−1. The changes of standard entropy for the same complexes were 6.68 and 27.15 kJ∙mol−1 respectively. The values of the thermodynamic parameters were calculated using the Van’t Hoff Equation (2) and the graph for UVBNB and βCD and βCD-MCT can be observed in Figure 9.
= −11.35 kJ∙mol−1. The changes of standard entropy for the same complexes were 6.68 and 27.15 kJ∙mol−1 respectively. The values of the thermodynamic parameters were calculated using the Van’t Hoff Equation (2) and the graph for UVBNB and βCD and βCD-MCT can be observed in Figure 9.
The ![]() values indicate the formation of a stable compound where the complex
values indicate the formation of a stable compound where the complex
![]()
Figure 8. Benesi-Hildebrand plot of 1/DF vs. 1/
[CD]
for both βCD and βCD-MCT at a fixed UVBNB concentration of 0.5 mmol∙dm−3.
concentration depends on the stoichiometry, since this is inversely proportional to the ratio host:guest in the complexation. The values of Gibbs free energy indicate a stable formation of the complexes.
UVBNB is a commercial optical brightener, their active compound is 2,5-Bis(5-tert- butyl-benzoxazol-2yl)-thiophene (concentration 13% v/v) and it is dissolved in a mixture of organic solvents and surfactants to achieve a final product soluble in water.
The stoichiometry studies on the complexation of UVBNB with βCD and βCD-MCT were also carried out using the continuous variation method (Job’s plot). Since UVBNB is a commercial product the results obtained show the overall ratio of complexation of all components that made up the UVBNB sample.
Figure 10 (Job’s plot) shows that stoichiometry for both CDs is 3:1 (host:guest). This stoichiometry could be owed to the contributions of complexation products between the CD and the organic compounds used to achieve the water solubility of the commercial sample of UVBNB: Since the UVBNB is a commercial product is not possible to determinate exactly which compounds are present in the suspension, despite the unknown contribution of every compound, the most important fact is that β-cyclodextrins improve significantly the fluorescence intensity of the FWA in solution.
The UVBNB is used to increase brightness and whiteness of some polymeric materials (such as cellulose in papermaking process), but organic solvents and surfactants are needed for the formulation in the commercial product in order to reach the solubility in water. The shift and the increase in the maximum fluorescence intensity is important because is possible to use inclusion complexation to improve the optical properties instead to the use organic solvents and surfactants. Some advantages using inclusion complexation of fluorescent whitening agents are: the shift of the maximum fluorescence intensity is located near to the wavelength where the brightness of the materials are measured; the complexation process can be useful to improve the optical properties using less optical brightener, which is usually expensive. Additionally, the compounds included in cyclodextrin cavity become more stable.
![]()
Figure 9. Reciprocal of temperature vs. ![]() for inclusion complex between UVBNB and βCD and βCD-MCT, according to van Hoff equation.
for inclusion complex between UVBNB and βCD and βCD-MCT, according to van Hoff equation.
![]()
Figure 10. A Job’s plot of the complexation of UVBNB with βCD and βCD-MCT in aqueous solution (
[Host]
+
[Guest]
= 0.5 mmol∙dm−3).
![]()
Table 2. Summary of the thermodynamic parameters obtained for the complexation of CDs and optical brighteners studied.
Table 2 is a summary results obtained for inclusion complex of βCD and βCD-MCT and fluorescent agents UVOB and UVBNB, stability and thermodynamic parameters. The larger values of
indicate higher concentration of complexes formed. The βCD was the better option for complexation with UVOB, it has the larger
and ∆Gº values (in absolute value), as an indication of better complexation process and stability. The results for
and ∆Gº values for UVBNB were very close together, slightly higher value for βCD, the magnitude of the values indicate a spontaneous and stable inclusion complex formation at the experimental conditions used. It is important to note that UVBNB is a commercial product of the fluorophore, so the 3:1 stoichiometry it is a result of complexation with the whole of organic compounds present in the solutions, nevertheless, the improvement of fluorescence intensity using cyclodextrins is very important in some industrial process, as a papermaking process because is possible to use less amount fluorescent compound with the same results in optical properties of the paper.
4. Conclusions
The study of the formation of inclusion compounds between cyclodextrins and fluorescent whitening agents was accomplished by the use of spectrofluorometry.
The fluorescence enhancement showed by the whitening compounds when forming complexes with cyclodextrins allowed the determination of the stoichiometry and the thermodynamic properties of such systems in solution. The equilibrium constant and the free energy of Gibbs of a βCD-1,8-ANS complex obtained by this approach agreed with values reported in the literature [26] .
All the inclusion complex systems studied (native and monochlorotriazinyl β-cyclo- dextrins with UVOB and UVBNB) showed spontaneous formation and stable products in solution at the experimental conditions employed in the study. The complexes of UVOB with βCD and βCD-MCT showed 1:1 (host:guest) stoichiometries using acetone/water (90/10 v/v) media. The complexes formed with UVBNB with both cyclodextrins, βCD and βCD-MCT, showed stoichiometries (3:1), when formed in aqueous media. This anomalous molar ratio was probably an average of the complexation of cyclodextrins with the fluorophore and the organic solvents as well as the surfactants present in the commercial sample used for the study (UVBNB).
Higher equilibrium constant values were observed for complexes formed with native β-CD, which indicated more stable compounds. KS values for modified cyclodextrins were lower. This could be owed to steric hindrance caused by the substituting groups on the cyclodextrin.
The complexation of water insoluble compounds, such as fluorescent whitening agents, with cyclodextrins could be an interesting approach for their application in aqueous media and improve additionally their fluorescent power.