Optical, Structural and Morphological Properties of Photocatalytic ZnO Thin Films Deposited by Pray Pyrolysis Technique ()
1. Introduction
Zinc oxide (ZnO) is an n-type semiconductor, has a hexagonal wurtzite structure, and has been extensively studied in recent years owing to its technical importance. ZnO is widely used as a functional material because it has a direct band gap of 3.37 eV, high electron mobility, and large exciton binding energy (~60 meV) at room temperature [1] . It has excellent chemical and thermal stability, low cost, richly abundant, environment-friendly, non-toxic nature, strong oxidizing power, high photosensitivity, and high resistance to radiation damage. ZnO has now received increasing attention and recognized as a promising material because of its vast potential in many scientific and industrial areas as a micro-electronic and opto-electronic devices such as light emitting diodes [2] , thin film transistors [3] [4] , Varistors [5] , solar cells [6] [7] [8] , gas sensors [9] [10] [11] [12] , and photocatalyst [13] [14] . And also, it has other applications, such as piezoelectric devices, spin based electronics or spintronics, luminescent materials, and transparent conductors [6] [8] [15] [16] . Till now ZnO thin films have been synthesized by various techniques including sol-gel spin coating method [4] , low pressure chemical vapor deposition (LPCVD) [7] [8] , Chemical Both Deposition (CBD) [12] , spray-pyrolysis [13] [14] , thermal evaporation [17] , pulsed laser deposition (PLD) [18] , metal organic chemical vapor deposition (MOCVD) [19] , sputtering [20] , molecular beam epitaxy [21] , and Hydrothermal Technique [15] , etc. Spray pyrolysis is widely used because of its simplicity, commercial viability, and potential for cost-effective mass production. The spray-pyrolysis technique has been successfully applied to synthesize ZnO thin films and analyzed their growing characteristics, atomic composition, surface morphology, microstructure and optical properties. Spray pyrolysis method is especially efficient in producing thin films, transparent, multi-component oxide layers of many compositions on various substrates, including glass. There are many factors affecting the physical properties of ZnO thin films, including ZnO solution concentration, solvent, substrate temperature, post deposition annealing, annealing atmosphere and film thickness. The novel results derived from this situation have encouraged the researchers to increase their interest in the field of chemically sprayed ZnO thin films. This is the reason why we focus our attention mainly on the contributions on chemically sprayed ZnO thin films.
In this article, our interest is focused on adsorption and photocatalytic ability for organic dye over ZnO catalyst in aqueous solution. We aim to synthesize promising ZnO nanofibers that behave as an efficient photocatalyst by low cost spray pyrolysis technique and effects are being made to characterize the ZnO nanofibers using structurally, morphologically and studied its photocatalytic activity.
2. Experimental Details
2.1. Materials and Chemicals
Zinc acetate dihydrate [Zn(CH3COO)2∙2H2O, Himedia, 99.5%], double distilled water, methylene blue (MB), Glass micro slide substrates with dimensions 75 mm × 25 mm × 1.2 mm (Blue star, Polar Industrial Corporation, India) were applied for deposition of ZnO thin films by simple spray pyrolysis technique at a substrate temperature of 350˚C.
2.2. Synthesis of ZnO Thin Films
The ZnO thin films were deposited on glass substrates at a substrate temperature of 350˚C by spray pyrolysis deposition technique. The ZnO precursor solution was prepared by dissolving 0.1 M of zinc acetate dihydrate [Zn(CH3COO)2∙2H2O, Himedia, 99.5%] in 25 ml deionized water. Before deposition the glass substrates were cleaned with detergent solution, diluted HCl and deionized water. Further, ultrasonic cleaning was carried out for 30 min in an ultrasonic bath and then rinsed in acetone for 10 min. Then the ultrasonically cleaned substrates were placed on a sample holder facing the air-atomizing nozzle at a fixed distance of 28 cm in the chamber. Compressed air was used as the carrier gas at the pressure of 2 bar. The prepared solution was sprayed (3 ml/min) onto the clean glass substrates. During spray pyrolysis, a precursor solution was sprayed as fine droplets on to a heated substrate. When the droplets reach the heated substrate, they spread out and undergo pyrolytic decomposition. Finally, the solid compounds react to become a new chemical compound. The possible chemical reaction that takes place on the heated substrate to produce ZnO thin film may be as follows [22] [23] .
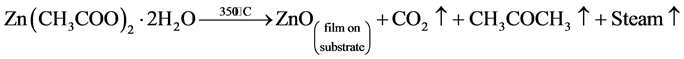
The deposited films were annealed at 450˚C for 1 hr in the muffle furnace using a ramp-rate of 10˚C per minute.
2.3. Characterization
The structural properties and phase identification of the films were done by using X-ray diffractometer with CuKα radiation (λ = 1.5406 Å) (Panalytical Xpert pro) for the 2θ range from 20˚ to 80˚ at room temperature. The surface morphology was studied with a scanning electron microscope (ZEISS, EVO-18). Stoichiometry of the film estimated using EDX. The UV-visible optical transmission and absorption spectra of ZnO thin films were recorded by UV-VIS spectrophotometer (UV-3092). The photocatalytic activity was evaluated by the degradation of MB under visible light irradiation and optical absorption spectrum of MB solution was measured using a UV-Vis spectrophotometer. The thickness of the film was measured by weight difference method using the relation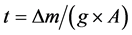 . Where, Δm is the mass difference measured using micro balance i.e. mass of the substrate measured after deposition and before deposition. It is taken in grams. “A” is the surface area of the film in cm2 and g is the density of deposited material (density of ZnO = 5.61 gm/cm3). The film thickness was found to be around 1.5 μm.
. Where, Δm is the mass difference measured using micro balance i.e. mass of the substrate measured after deposition and before deposition. It is taken in grams. “A” is the surface area of the film in cm2 and g is the density of deposited material (density of ZnO = 5.61 gm/cm3). The film thickness was found to be around 1.5 μm.
3. Results and Discussion
3.1. Structural Properties of the ZnO Thin Films
Figure 1, shows the phase identification and crystal structure of the ZnO thin films of the as prepared and annealed at 450˚C for 1 hr. These films are found to be polycrystal-
![]()
Figure 1. XRD patterns of the ZnO thin films.
line in nature and exhibit single phase hexagonal wurtzite structure. The observed XRD patters are compared with standard JCPDS data file (File Nos. 890510). The observed 2θ values are in good agreement with the standard ones, and having a hexagonal wurtzite structure with lattice constants a = 3.248 Å and c = 5.205 Å. It is clearly seen from the figure that the crystallinity of ZnO film increases with the annealing temperature. The films showed several diffraction peaks at 2θ = 31.808˚, 34.435˚, 36.250˚, 47.538˚, 56.602˚, 62.795˚, 67.945˚, and 69.120˚ having the inter planar spacing of 2.813 Å, 2.605 Å, 2.478 Å, 1.626 Å, 1.480 Å, 1.379 Å, 1.358 Å and 1.358 Å with orientation of (100), (002), (101), (102), (110), (103), (112), and (201) planes respectively. The ZnO thin films exhibit three prominent peaks of (100), (002), and (101) plane orientations. Again, some low intensity peaks corresponding to the orientations of (102), (110), (103), (112), and (201) are also present. The annealing temperature plays an important role on the surface reactions and species mobility. The lattice constants “a” and “c” of the hexagonal wurtzite structure of ZnO films can be calculated and was found to be a = b = 3.252 Å and c = 5.204 Å by using the given below relation [24] .
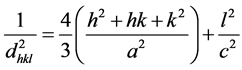 (1)
(1)
The crystallite size has been evaluated from peak profile using full width at half maximum (FWHM) of the corresponding diffraction peak using Debye-Scherrer’s formula [25] .
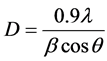 (2)
(2)
where “θ” is the Bragg diffraction angle, β is the FWHM (taken in radians) of the (002) plane orientation diffraction peak and λ is the wavelength of the incident X-ray (λ = 0.15406 nm). The lattice strain (ε) and dislocation densities (δ) were calculated using the relation given below [25] .
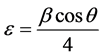 (3)
(3)
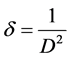 (4)
(4)
The dislocation density (δ), defined as the length of dislocation lines per unit volume of the crystal and it gives more information about the crystal structure. Better the crystallization smaller will be the dislocation density. The crystallite size found to increase with increase in annealing temperature, whereas the strain and dislocation density are found to decrease.
The preferential growth orientation was determined using a texture coefficient TC(hkl). This factor can be calculated using the following relation [26] .
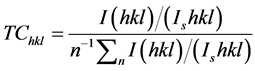 (5)
(5)
where TC(hkl) is texture coefficient, I(hkl) is the measured XRD intensity of a plane (hkl) obtained from the films, Is(hkl) is the standard intensity of the plane (hkl) taken from the JCPDS data, and n is the number of diffraction peaks considered. The variations of texture coefficient calculated for the three main diffraction peaks ((100), (002) and (101)) of wurtzite ZnO, are listed in Table 1. The texture coefficient for the (002) orientation has been found to increase from 1.476 to 1.622 with annealing temperature. The value of the texture coefficient indicates a sample with randomly oriented crystallite presents TC(h, k, l) = 1, while the larger value, the larger abundance of crystallites oriented at the (hkl) direction. Thus, in the present investigation, with increase in annealing temperature, the crystallinity along (002) plane improves.
3.2. Surface Morphology and Composition Analysis of Zno Thin Films
Figure 2, shows the surface morphology of as deposited and annealed ZnO thin films. SEM micrographs reveals that the thin films were found to be crack free, having uniform texture the substrate and the films were found to be nano fiber like structure. The average diameter of the fibers for the as deposited and annealed at 450˚C was found to be approximately 390 nm, and 400 nm respectively. Ilican et al. (2006) also reported the indium-doped zinc oxide nanofibers with uniform diameter of 200 nm [26] . In our investigation the average diameter of the fibers increases with annealing temperature, this is probably due to agglomeration during annealing process, and producing thicker
![]()
Table 1. Microstructural parameters of ZnO thin film.
TC is the texture coefficient; FWHM is the full width at half maximum.
diameter of fibers. Similarly it has been observed that the annealing temperature has profound influence on the diameter of the nano fibres [27] . Stoichiometry and Compositional analysis of ZnO thin film was carried out by the energy dispersive X-ray spectrometer (EDX) shown in Figure 3. The EDX analysis confirmed the presence of Zn and O elements in the ZnO film.
3.3. Optical Studies
The optical properties of bulk materials and thin films that are very important for optoelectronic applications, the properties include absorbance (A), transmittance (T), and reflectance (R), which characterize the interaction of incident radiation with a particular coating of material. These properties are also related to intrinsic properties of films such as coefficient of absorption (α), extinction coefficient (k), refractive index (n),
![]()
Figure 2. SEM images of ZnO thin films: (a) as deposited, (b) annealed at 450˚C.
![]()
Figure 3. EDX analysis of ZnO thin film annealed at 450˚C.
band gap energy and optical conductivity. In the present investigation, we calculated the absorbance and transmittance as a function of the wave length at room temperature. From the above parameters the reflectance of the films can be estimated using the following relation [28] .
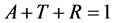 (6)
(6)
The optical transmittance and absorbance of ZnO films of as prepared and annealed at different temperatures shown in Figure 4(a) and Figure 4(b). Absorption spectrum indicates that the absorption of light in visible region is very low and greater in the UV region. Absorption spectra of ZnO thin film shows that the absorption edge is slightly moved towards longer wavelength side (red shift) with increase in annealing temperature
![]()
![]()
Figure 4. (a) Optical transmittance spectra of ZnO thin films, (b) Optical absorption spectra of ZnO thin films.
due to the change in band gap of ZnO thin film due to change in crystallite size. ZnO film exhibits high transmittance (>85%) in the visible region, the transmission of ZnO film is high over large wavelengths. This suggests that the produced film indicates a good optical quality due to the low scattering/ absorption losses. In nanocrystalline semiconductors, the following equation has been obtained to relate the absorption coefficient to incident photon energy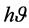 . The absorption coefficient
. The absorption coefficient 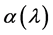 has been calculated using Lambert’s law [29] [30]
has been calculated using Lambert’s law [29] [30]
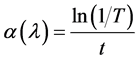 (7)
(7)
where t is the thickness of film. The extinction coefficient can be obtained from the
relation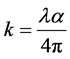 , where
, where ![]() is the wavelength of the incident radiation impinging on
is the wavelength of the incident radiation impinging on
the film. The extinction coefficient as a function of wavelength and it is observed that the excitation coefficient decreases with the increase of annealing temperature. This behavior of the absorption coefficient ![]() and the extinction coefficient (k) is similar to the optical absorption behavior. The Optical energy gap Eg and absorption coefficient
and the extinction coefficient (k) is similar to the optical absorption behavior. The Optical energy gap Eg and absorption coefficient ![]() are related by the equation [29] [30]
are related by the equation [29] [30]
![]() (8)
(8)
where ![]() is the photon energy,
is the photon energy, ![]() is the absorption coefficient, Eg is the optical band gap and B is the slope of the tauc edge, called the band tailing parameter and the exponent “r” depends on the type of optical transition, which is 1/2 for direct allowed transition and 2 for indirect allowed transition. Whether the film has direct or indirect band gaps will be determined from the linearity of the plots of
is the absorption coefficient, Eg is the optical band gap and B is the slope of the tauc edge, called the band tailing parameter and the exponent “r” depends on the type of optical transition, which is 1/2 for direct allowed transition and 2 for indirect allowed transition. Whether the film has direct or indirect band gaps will be determined from the linearity of the plots of ![]() versus
versus ![]() and
and ![]() versus
versus ![]() respectively. Figure 5, shows the tauc plots of ZnO thin films. The band gap values have been calculated by extrapolating the straight line portion of the curve of
respectively. Figure 5, shows the tauc plots of ZnO thin films. The band gap values have been calculated by extrapolating the straight line portion of the curve of ![]() against
against ![]() to meet the x-axis. The band gap of the ZnO film was obtained as a direct band gap by the optical method, which was described above. Table 2 gives the values of Eg of the ZnO thin films of as deposited and annealed at different temperatures. The band gap energy of the films was found to decreases with increase of annealing temperature. The change in the values of band gap energy may attribute to a decrease of the optical band gap due to interatomic distances with increasing annealing temperature on ZnO film [20] . The tail of the absorption edge is exponential, it indicates the presence of localized states in the band gap energy. The absorption edge gives a measure of the band gap energy and the exponential dependence of absorption coefficient
to meet the x-axis. The band gap of the ZnO film was obtained as a direct band gap by the optical method, which was described above. Table 2 gives the values of Eg of the ZnO thin films of as deposited and annealed at different temperatures. The band gap energy of the films was found to decreases with increase of annealing temperature. The change in the values of band gap energy may attribute to a decrease of the optical band gap due to interatomic distances with increasing annealing temperature on ZnO film [20] . The tail of the absorption edge is exponential, it indicates the presence of localized states in the band gap energy. The absorption edge gives a measure of the band gap energy and the exponential dependence of absorption coefficient ![]() on photon energy near the band edge for many materials. This dependence is given by Urbach F., 1953 [31] .
on photon energy near the band edge for many materials. This dependence is given by Urbach F., 1953 [31] .
![]() (9)
(9)
where αo is a constant and Eu is Urbach energy, which is the width of the tail of the localized states corresponding to the optical transition between localized states adjacent to the valence band and extended state in the conduction band which is lying above the
![]()
Figure 5. Plots of (αhυ)2 versus photon energy (hυ) of ZnO thin films.
![]()
Table 2. Optical parameters of the ZnO thin film.
Eg is the band gap energy, Eu is the urbach energy, Eo is the oscillatory energy, Ed is the dispersion energy, M−1 and M−3 are the moments; ![]() is the static dielectric constant,
is the static dielectric constant, ![]() is the oscillator wavelength, So is the oscillator length strength.
is the oscillator wavelength, So is the oscillator length strength.
mobility edge. The plot of lnα vs. photon energy ![]() plots for ZnO thin films of is shown in Figure 6. The values of Eu, were obtained by taking the reciprocal of the slope of the linear region of the plots of lnα versus
plots for ZnO thin films of is shown in Figure 6. The values of Eu, were obtained by taking the reciprocal of the slope of the linear region of the plots of lnα versus ![]() and are listed in Table 2. In our observation, the increase in Urbach energy may be attributed to the increase of thermal induced structural disorder of the film within this temperature range.
and are listed in Table 2. In our observation, the increase in Urbach energy may be attributed to the increase of thermal induced structural disorder of the film within this temperature range.
Figure 7, shows the plot of refractive index (n) versus wavelength (nm) of ZnO thin films and it indicates that the refractive index decreases with increase in wavelength. Refractive index of the films was calculated from the following relation [32] .
![]() (10)
(10)
where R is reflectance and k is extinction coefficient. The refractive index dispersion of the ZnO films studied can be fitted by the single-oscillator model (described by Wemple and DiDomenico, 1971). The dispersion plays an important role in the research of optical materials, because it is a significant factor in optical communication and in designing devices for spectral dispersion. The result of refractive index dispersion below the inter band absorption edge corresponds to the fundamental electronic excitation spectrum, according to the Wemple-DiDomenico single oscillator model, the refractive index is related to photon energy through the relationship [33] [34] .
![]() (11)
(11)
where ![]() and
and ![]() are single-oscillator parameters. Values of Eo and Ed can be deter- mined from the slope
are single-oscillator parameters. Values of Eo and Ed can be deter- mined from the slope ![]() and intercept
and intercept ![]() on vertical axis of the curves of (n2 − 1)−1 vs.
on vertical axis of the curves of (n2 − 1)−1 vs. ![]() by fitting a linear function to the smaller energy data shown
by fitting a linear function to the smaller energy data shown
![]()
Figure 6. Plots of lnα versus photon energy (hυ) of ZnO thin films.
![]()
Figure 7. The refractive index plots of ZnO thin films.
In Figure 8 and they are given in Table 2. The oscillator energy ![]() is average excitation energy for electronic transitions. The dispersion energy
is average excitation energy for electronic transitions. The dispersion energy ![]() which is measure of the average strength of inter band optical transitions or the oscillator strength are single oscillator parameters. We found that the
which is measure of the average strength of inter band optical transitions or the oscillator strength are single oscillator parameters. We found that the ![]() values of the deposited film is related empirically to the lowest direct band gap by
values of the deposited film is related empirically to the lowest direct band gap by![]() .
. ![]() values decreased with an increase in annealing temperature. According to the single-oscillator model, the single oscillator parameters
values decreased with an increase in annealing temperature. According to the single-oscillator model, the single oscillator parameters ![]() and
and ![]() are related to the imaginary part of the complex dielectric constant. The static (zero frequency) refractive index
are related to the imaginary part of the complex dielectric constant. The static (zero frequency) refractive index ![]() and the static dielectric constant
and the static dielectric constant ![]() were calculated using Equation (11). The calculated values of the oscillator energies
were calculated using Equation (11). The calculated values of the oscillator energies![]() , the dispersion energies
, the dispersion energies![]() , and εs for the ZnO thin film are summarized in Table 2. The refractive index is represented by a single Sellmeier oscillator at low energies and the refractive index was also analyzed to determine the long wavelength refractive index
, and εs for the ZnO thin film are summarized in Table 2. The refractive index is represented by a single Sellmeier oscillator at low energies and the refractive index was also analyzed to determine the long wavelength refractive index![]() , average inter band oscillator wavelength
, average inter band oscillator wavelength ![]() and the average oscillator length strength
and the average oscillator length strength ![]() parameters, for the ZnO thin films. These parameters can be determined by using the following equation [35] .
parameters, for the ZnO thin films. These parameters can be determined by using the following equation [35] .
![]() (12)
(12)
where![]() , and
, and ![]() values were calculated from intercept and the slope of the linear region of the plots of
values were calculated from intercept and the slope of the linear region of the plots of ![]() vs
vs ![]() and are given in Table 2.
and are given in Table 2.
Rearranging of Equation (12), gives [36]
![]() (13)
(13)
![]()
Figure 8. The plots of ![]() versus (hυ)2 ZnO thin films.
versus (hυ)2 ZnO thin films.
where
![]() (14)
(14)
The ![]() values for ZnO films were obtained using above equation and are given in Table 2. The M−1 and M−3 moments of the imaginary part of the optical spectrum can be obtained from the relations [33] :
values for ZnO films were obtained using above equation and are given in Table 2. The M−1 and M−3 moments of the imaginary part of the optical spectrum can be obtained from the relations [33] :
![]() (15)
(15)
The M−1 and M−3 moments were calculated using Equation (15) and the obtained values are given in Table 2. The M−1 and M−3 moments changed due to the formation coordination of complex. It is found that M−1 and M−3 values increase with increase in annealing temperature.
3.4. Evaluation of Photocatalytic Activity
Figure 9(a), shows optical absorption spectrum of MB solution, before and after degradation under ZnO thin films. It revealed that the absorbance intensity of MB decreases obviously under visible light irradiation, which indicates that MB has been photodegraded. However, without light illumination, in the presence of ZnO film, and illumination in the absence of ZnO film does not result in the photocatalytic degradation of MB solution. A 200 W tungsten filament bulb was used as a light soure. The standard solution of methylene blue with the concentration of 1 × 10−6 M was used as a model pollutant organic dye to determine the photocatalytic efficiency of the prepared photocatalist. Photodegradation experiment was carried out by the ZnO film immersed into 15 ml of MB solution and it was stirred in the dark in order to reach adsorption desorption equilibrium between MB and dissolved oxygen before irradiation and the beaker was kept in a chamber under visible light irradiation for different exposition times. Optical absorption spectrum of MB solution was measured using a UV-Vis spectrophotometer. The degradation efficiency was calculated using the following equation [37]
![]() (16)
(16)
where Co and C represent the initial concentration, after the adsorption-desorption equilibrium and at the reaction time (t) concentration of MB solution, respectively. Ao represents the initial absorbance, and A represents the changed absorbance of the MB solution at the characteristic absorption wavelength of 666 nm. The performance of degradation of as deposited and annealed ZnO films were reached to 90% and 93% after 240 min respectively. The highest catalytic activity was obtained by annealed ZnO catalyst due to the lower band gap energy. In addition, the relationship between the irradiation time and degradation reaction rate obeys the first-order reaction kinetic model, which could be well explained in terms of the Langmuir-Hinshelwood mechanism [37] . The kinetics can be expressed as
![]() (17)
(17)
where C and Co, represent changed concentration and initial concentration of MB, ![]() and t is the apparent rate kinetic constant and irradiation time, respectively. The
and t is the apparent rate kinetic constant and irradiation time, respectively. The
plot of ![]() versus irradiation time (t) for ZnO is shown in Figure 9(b).
versus irradiation time (t) for ZnO is shown in Figure 9(b).
Obtained kinetic plots are linear, which confirms that the photodegradation reaction of MB on ZnO is well fitted to the pseudo-first order reaction kinetics. The photocatalytic reaction rate constant ![]() value for catalyst was estimated from the slop of this
value for catalyst was estimated from the slop of this
![]()
![]()
Figure 9. (a) Absorption spectra of MB catalyzed at room temperature in the presence of ZnO film annealed at 450˚C, (b). The first order kinetics of the degradation of Methylene Blue dye with irradiation time.
linear plots and it were found to 0.0097 min−1 and 0.0111 min−1 for as deposited and annealed films respectively. It is clear that the catalytic activity higher for annealed ZnO. Therefore, the ZnO nanofibers with a low band gap can significantly increase the catalytic activity. However, the kinetic constant of the catalyst become smaller with greater band gap, because the opacity and light scattering of the ZnO nanofibers decrease the absorption of incident light and decrease catalytic active sites.
4. Conclusion
Visible light responsive zinc oxide films were deposited on glass substrate at a temperature of 350oC by chemical spray pyrolysis technique. The first order kinetic rate constant has been determined from the MB degradation measurement. ZnO film exhibits high catalytic activity for lower band gap energy. The lattice constants of hexagonal wurtzite structure ZnO films were calculated as 3.252 Å and 5.204 Å. Crystallite size has been found increase from 15 nm to 24 nm with annealing temperature. The SEM images of the films shown nano fiber like structure morphology and the average diameter of the fibers were ~390 nm and ~400 nm as deposited and annealed at 450˚C respectively. The films exhibit low absorbance in the visible/near infrared (NIR) region from ~390 nm to 1000 nm. The direct optical band gap is 3.31 eV for deposited and 3.26 eV for annealed films. The oscillator energy (Eo), and the dispersion energy (Ed) of the ZnO thin films were determined. The values of oscillator energy (Eo) decrease, and the M−1 and M−3 optical moments increase with increasing annealing temperature. The long wavelength refractive index![]() , average inter band oscillator wavelength
, average inter band oscillator wavelength ![]() and the average oscillator length strength
and the average oscillator length strength ![]() parameters are found to be function of annealing temperature. The optical constants (refractive index n, extinction coefficient k, band gap energy and dielectric constant) of the as-deposited and annealed films were analyzed from the transmittance and reflectance spectra. Based on the observed data ZnO thin films are found to be a promising candidate for opto-electronic device and potential applications in the field of environmental remediation and photocatalysis.
parameters are found to be function of annealing temperature. The optical constants (refractive index n, extinction coefficient k, band gap energy and dielectric constant) of the as-deposited and annealed films were analyzed from the transmittance and reflectance spectra. Based on the observed data ZnO thin films are found to be a promising candidate for opto-electronic device and potential applications in the field of environmental remediation and photocatalysis.
Acknowledgements
One of the authors D. Komaraiah, thank the University Grants Commission, New Delhi for awarding the SRF, under the UGC scheme of RFSMS and also the authors R. Sayanna and M.V. Ramana Reddy, thank the UGC-UPE-FAR-OU for providing financial assistance to carry out this work.