Application of Carbon Dioxide and Polyacrylamide in Extracting Magnesium from Brine by Precipitation Method ()


1. Introduction
Magnesium is an important industrial raw material, which can not only be used as a reductant to recover metals like titanium, zirconium, uranium and beryllium, but also can be used to make fireworks, flash powder, fireproof materials, etc. With structural properties similar to aluminum, magnesium can be used as an alloy material for aircrafts and missiles. In addition to the industrial sectors, magnesium is also used in medical, sports, chemical reagent manufacturing and other areas.
Magnesium is a widely-distributed element, which was obtained mainly by ore smelting in the past. In recent years, with the acceleration of the marine resources development, seawater has also become an important source of magnesium. Magnesium content in seawater and brine ranks only next to sodium and chlorine, and thus is worth exploring. Meanwhile, with the rapid development of seawater desalination industry, extraction of magnesium from desalination brine has also gained researchers' attention. Since most of elements contained in desalination brine are higher in concentration than those in normal seawater, obtaining equivalent amounts of chemical resources from desalination brine is easier than extracting from normal seawater. In addition, chemical resource extraction from desalination brine can greatly save investment and engineering costs because it does not require additional pretreatment equipment such as seawater intake, chlorination facilities, and so on.
At present, magnesium is extracted from brine mainly by precipitation method [1]. Alkali is added into the brine to produce magnesium hydroxide precipitate to separate magnesium from the brine through phase separation, which is the most rapid and effective method [2]. In this process, two core problems need to be addressed: one is the interference by calcium ions; and the other is the improvement of magnesium ion precipitation efficiency. In this study, the extents to which these two difficulties can be resolved are investigated through experiments using two chemicals, carbon dioxide (CO2) and polyacrylamide (PAM).
2. Materials and Methods
2.1. Experimental Materials
(1) CO2(99%); polyacrylamide (A.R.)
(2) Desalination brine was collected from the brine outfall for desalination project of Liuheng Water Services Ltd. in Zhoushan City, Zhejiang Province, China.
2.2. Experimental Methods
2.2.1. Removal of Calcium
Preliminary experiments have shown that magnesium hydrate obtained by precipitation had poor purity when desalination brine was directly used as the raw material, with a calcium content of about 3% - 7%. If a solution removed of calcium was operated previously, calcium content would be reduced to less than 1%. In an alkaline environment, carbon dioxide was allowed to preferentially react with calcium ion in the desalination brine, then the prepared decalcified mother liquor containing low calcium ion was reacted with alkali to produce the desired product magnesium hydrate. In this way, the purity of magnesium precipitate could be improved [3]-[5].
In the first set of experiments, 200.00 mL of brine was placed in a three-necked flat-bottom flask; speed of magnetic stirrer was controlled at 600 rpm; pH meter was inserted below the liquid level; and pH value was recorded after the reading stabilized. Next, carbon dioxide (flow rate of about 0.5 L∙min−1) was passed through the solution; meanwhile, 2.5 mol∙L−1 sodium hydroxide solution was added dropwise. Timing started when the pH of the system changed to 8 ± 0.2. After reacting for certain times (1 min, 2 min, 3 min, 4 min and 5 min), and at the same time, carbon dioxide and sodium hydroxide delivery were ceased. Afterwards, stirring was continued for 1 h, and then the mixture was allowed to stand for a certain time. After filtering through quantitative paper, concentrations of remaining calcium ion and magnesium ion in the clear liquor were measured and the amounts of substance were calculated, which were compared to the concentrations and molar amounts of calcium ion and magnesium ion in desalination brine before reaction to calculate the calcium removal rate and magnesium loss rate.
In the second set of experiments, conditions were set identically with the first set of experiments, except that stirring was terminated immediately after completion of reaction. After letting stand for a while, Ca2+ removal rate and Mg2+ loss rate were calculated.
2.2.2. Preparation of Magnesium Precipitate
Decalcified mother liquor was added with certain amounts of precipitant sodium hydroxide solution and coagulant aid PAM solution, then reacted, stood still and filtered to give magnesium hydroxide product. By controlling relevant parameters at different levels, the influences of sodium hydroxide addition amount, PAM addition amount, addition order of sodium hydroxide and polyacrylamide and standing time on precipitation effect were investigated.
100.00 mL of decalcified mother liquor was accurately pipetted and transferred to a 250 mL beaker used as the reaction vessel. pH and temperature of the reaction system were adjusted and measured. After certain amounts of sodium hydroxide and PAM solutions were added, pH and temperature, which stabilized after reaction, were recorded accurately. After letting stand for a while, the mixture was filtered, magnesium ion and calcium ion contents in the filtrate were determined. Optimal proportion was confirmed by continuously changing the molar amount of alkali solution added and the concentration of PAM.
(1) Effect of concentration of PAM solution on magnesium precipitation rate
PAM solutions with mass concentrations of 0.1%, 0.2% and 0.3% were prepared separately while other parameters remained unchanged. Through reaction, precipitation, standing, filtration, titration and calculation, the appropriate concentration of PAM solution was determined.
(2) Effect of addition amount of hydroxide ions on magnesium precipitation rate
Calcium ion and magnesium ion contents in decalcified mother liquor were accurately measured and theoretical molar amount of hydroxide ion required for magnesium ion in 100.00 mL of decalcified mother liquor was calculated, based on which the molar amount of actually added hydroxide ion was changed continuously. Through reaction, precipitation, standing, filtration, titration and calculation, the appropriate molar amount of hydroxide ion was determined.
(3) Effect of standing time on magnesium precipitation rate
After formation of magnesium hydroxide precipitate, contents of remaining calcium ion and magnesium ion in solution were determined at different standing times, in order to compare the magnesium precipitation rates at different standing times and analyze the effect of standing time on magnesium hydroxide yield.
2.2.3. Analytical Determination Method
Titration method was used to determine calcium ion and magnesium ion concentrations [6].
3. Results and Discussion
3.1. Preparation of Decalcified Mother Liquor
Calcium and magnesium were very similar elements in desalination brine. Carbon dioxide could separate these two elements just because calcium carbonate had a weaker soluability than magnesium carbonate [7]. In this study, we selected the most common temperature 25˚C as the elementary reacting condition, carbon dioxide was injected as a sufficient reagent to remove calcium from the desalination brine. As can be seen from Table 1, controlling pH within 7.8 - 8.4 was the primary condition for not less than 90% yield of calcium carbonate and less than 5% yield of Magnesium hydrate. If pH was too low, the resulting calcium carbonate would be easily decomposed; and if pH was too high, calcium carbonate would be easily produced and magnesium loss rate would be high. Compared with pH, reaction time was more important to decide magenium loss rate. It was easy to get a calcium removal rate more than 90%, but only reaction time was with 3 - 4 minutes could we get a magnesium loss rate less than 5%.
During the carbon dioxide inlet process, calcium carbonate was produced first. As the reaction proceeded, calcium ion concentration in the system decreased gradually. When it decreased to less than KspCaCO3/[ ], carbonate started to react with magnesium ion. So when the reaction time was prolonged to 5 min, the magnesium loss rate increased significantly.
], carbonate started to react with magnesium ion. So when the reaction time was prolonged to 5 min, the magnesium loss rate increased significantly.
Molecular collision was a key factor in calcium removal. Long duration of molecular collision was a major cause of high calcium removal rate and magnesium loss rate. Magnetic stirring promoted the collision. Stirring time of 3 - 4 min was appropriate to ensure that the high-energy molecules transferred part of the energy to low-energy molecules. Weakest bond received the energy and broke to form effective collision, and thereby produced new substances. When the effective collision was sufficient, the length of precipitate standing time had little effect on the precipitation of calcium and magnesium.
3.2. Magnesium Extraction by Precipitation Method
3.2.1. Effect of PAM Solution Concentration on Magnesium Precipitation Rate
The amide groups on PAM were interacted by hydrogen bond and then adsorbed the sol particles. After adsorption, PAM chain segments rotated and moved, that is, bridging effect aggregated solid particles together to form precipitate. Therefore, the use of PAM would facilitate the production of magnesium hydrate precipitate.
As can be seen from Figure 1, when the same molar amount of hydroxyl ions was added, the magnesium precipitation rates for the reaction systems added with 0.1% and 0.2% PAM were close to that for the reaction system without PAM delivery. This indicated that PAM did not prompt more magnesium in the reaction system to react with hydroxyl anion. In the presence of PAM, the yield did not increase significantly.
In addition, it can also be found from Figure 1 that when the ratio of molar amount of actually added hydroxyl ions to the theoretical value was the same, the magnesium precipitation rate was between 70.48% - 75.66% in the presence of 0.3% PAM, which was less than that in the PAM-free reaction system (greater than 77%). When
![]()
Table 1. Calcium removal rate and magnesium loss rate under different conditions.
![]()
Figure 1. Effect of PAM concentration on magnesium precipitation rate.
the mass concentration of PAM solution reached 0.3%, the solution had high viscosity, which was mixed slowly and expanded moderately in the reaction system, thus affecting the results. Comprehensive analysis of Figure 1 and Figure 2 showed that the PAM solution well aided the coagulation when its concentration was controlled within 0.1% - 0.2%.
3.2.2. Effect of Sodium Hydrate Delivery Amount on Magnsium Precipitation Rate
As can be seen from Table 2, when the ratio of nOH-actual to nOH-theoretical was 111.8%, magnesium hydrate yield was high. When the ratio of nOH-actual to nOH-theoretical increased to 130%, yield of Magnesium hydrate decreased instead; and at that time, calcium proportion in the precipitate rose.
3.2.3. Effect of Standing Time on Magnesium Precipitation Rate
Figure 3 shows changes in magnesium precipitation rate at different standing times. A. Magnesium hydrate yield was 50.25% - 59.15% at a standing time of 13 hours; and was 42.74% - 60.95% at a standing time of 18 hours. Moreover, the precipitation rate fluctuated widely, indicating insufficient standing and insufficient precipitate production. At a standing time of 60 h, yield was 70.48% - 77.22% when the addition amount of hydroxide ion was 101.5% - 130.2% of the theoretical value. At a standing time of 65 h, yield ranged from 71.48% to 74.40% when the addition amount of hydroxyl anion was 111.4% - 120.2% of the theoretical value, which was still slightly lower than that at a standing time of 65 h. At a standing time of 72 h, yield ranged 72.48% - 75.48%
![]()
Figure 2. Calcium and magnesium ratio in precipitation over hydroxyl ion dosage.
![]()
Table 2. Changes in magnesium precipitation rate with hydroxide ion delivery.
![]()
Figure 3. Effect of product standing time on magnesium precipitation rate.
when the addition amount of hydroxide ion was 111.4% - 120.2% of the theoretical value. Analysis of precipitation rates at standing times found that extension of standing time contributed little to the increase of precipitation yield at those times.
In addition, calcium ion did not show significant further reduction with increasing molar amount of hydroxide ion addition, indicating that the amount of calcium impurities in the product did not increase significantly. This was because after decalcification treatment, calcium ion concentration was low in the reaction material. Moreover, 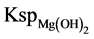 (5.6 × 10−12, 25˚C) was far smaller than
(5.6 × 10−12, 25˚C) was far smaller than 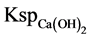 (5.5 × 10−6, 25˚C). Thus, magnesium hydrate precipitate was produced preferentially than calcium hydroxide.
(5.5 × 10−6, 25˚C). Thus, magnesium hydrate precipitate was produced preferentially than calcium hydroxide.
4. Conclusions
(1) 0.1% - 0.2% PAM solution is added and interacted with the hydrogen bonds in the system to produce bridging effect and promote the precipitation of magnesium hydrate colloid. However, the yield of magnesium hydrate does not improve significantly. When the concentration of PAM solution is low, the molar addition amount of sodium hydroxide solution is a major influencing factor of magnesium hydrate yield. When the ratio of nOH-actual to nOH-theoretical is within a 110% - 130% range, magnesium hydrate is largely produced; magnesium precipitation rate is higher than 73%. At the moment alkali solution is added to the decalcified mother liquor, the concentration product of magnesium ion and hydroxyl anion is far greater than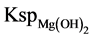 . Magnesium hydrate is produced and hydroxyl anion addition disrupts the kinetic stability of the system, thereby promoting inter- particle collisions. Particles lose anti-coagulation stability and coalesce together after collisions, resulting in enlarged particle size, decreased velocity and eventually fomed a kinetically unstable system. Magnesium hydrate particles growed larger and were gradually separated and precipitated from the decalcified mother liquor.
. Magnesium hydrate is produced and hydroxyl anion addition disrupts the kinetic stability of the system, thereby promoting inter- particle collisions. Particles lose anti-coagulation stability and coalesce together after collisions, resulting in enlarged particle size, decreased velocity and eventually fomed a kinetically unstable system. Magnesium hydrate particles growed larger and were gradually separated and precipitated from the decalcified mother liquor.
Magnesium in the desalination brine is obtained using sodium hydroxide as the precipitant. When the molar amount of hydroxyl anion is excessive, i.e. between 110% - 130%, and standing time of precipitate is 60 h, yield of magnesium hydrate exceeds 70%.
Calcium ion in the desalination brine can be effectively removed using carbon dioxide, which thereby improves the purity and reaction efficiency of the magnesium hydrate precipitate. Application of carbon dioxide in extracting magnesium from the desalination brine enables reduction of greenhouse gas emissions while alleviating the environmental problems brought by brine discharge [8] [9].
Acknowledgements
This study was supported by The National Natural Science Foundation of China (No. 41106066 and No. 41506126).