Factors Affecting the Reductive Properties of the Core-Shell SiO2-Coated Iron Nanoparticles ()
1. Introduction
Since 1990s, the zero-valent iron (ZVI, Fe0) has been widely used in wastewater treatment based on its relatively cheap price and easy availability [1] - [3] . However, a recent review summarized the general limitations and countermeasures of the ZVI technology [4] . The major drawback is the low intrinsic reaction rate due to mass transfer limitations imposed by the available surface, which is aggravated by gradually increasing passivation as iron corrosion products accumulate on the nZVI grains over time [5] . Nowadays, researchers pay attention to the performance and potential application of nZVI (particles size < 100 nm). The specific surface area of the nanoparticles (10 - 100 nm) is 10 times larger than that of micron-scale particles and the reactivity is significantly enhanced [6] . Over the past decades, nZVI has been considered to possess promising potential in environmental remediation because of their high reactivity as a reducing agent, and ability to generate Reactive Oxygen Species (ROS) through the Fenton reaction [7] [8] . However, the agglomeration of nZVI is one of the most fatal shortages of this process, which results in the rapid inactivation of chemical reactivity. Therefore, modification of the surface of nZVI to reduce agglomeration and prevent oxidation of ZVI is necessary. Two main methods are generally adopted. One of them is the use of surfactants and coatings so as to increase the repulsive forces between the nZVI [9] . Among various coatings, SiO2 coating has attracted considerable attention because of its stabilization and easy preparation. It had been reported that silica was a suitable coating agent which prevented particles aggregation through electrosteric stabilization. In addition, silica-coated particles were not sensitive to different ionic strengths, which suggested its suitability for subsurface applications [10] . Recent studies indicated that the SiO2-nZVI improved the antioxidation abilities and reducing capacity of iron nanoparticles. In most studies, the preparation and coating of nanoparticles are generally treated as two or more independent process. However, the complex synthetic method usually consumed more time and may cause the nZVI particles corrosion when they explored to the air [11] . For this reason, some studies tried to use one-step method to synthesize the SiO2 coating nanoparticles [12] . However, many factures probably influenced the effect of SiO2 coating in preparation process.
In this study, we optimized the preparation process of producing SiO2-nZVI. The nanoparticles which were prepared under different conditions were used to dye decolorization study. To obtain more details about SiO2-nZVI, the characteristics were studied by Transmission Electron Microscope (TEM) and powder X-Ray Diffraction (XRD). The results showed that the SiO2 generated a complete network structure out of nZVI particles. The nanoparticles obtained well stability characteristic and had high activity to decolorize dye compound. Therefore, SiO2-nZVI had a great potential for treatment of textile wastewater.
2. Materials and Methods
2.1. Chemicals
Ferric chloride hexahydrate (FeCl3∙H2O), potassium borohydride (KBH4), tetraethyl orthosilicate (TEOS), ethanol (EtOH), polyethylene glycol (PEG 400) and Malachite green were procured from the Guangfu Fine Chemical Research Institute (Tianjin, China). All other chemicals were of analytical grade or the highest quality. Malachite green was selected for the decolorizing tests.
2.2. Preparation of SiO2-nZVI
SiO2-nZVI was artificially synthesized by using One-Step method in this study. Ferric iron was reduced by borohydride according to the following equation:
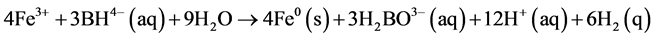
First, FeCl3∙6H2O (0.436 g) was diluted with the solution of ethanol/distilled water (7:3, 30 ml) and stirred in a serum bottle at room temperature. Then, 0.5 ml TEOS was added to the iron solution. The mixture was agitated in a magnetic stirrer at room temperature and this process required the protection of nitrogen gas. Black iron nanoparticles formed when 0.537 mol/l KBH4 was added under an accurate control of flow rate (2 ml/min). The mixture was reacted at room temperature under a nitrogen flow for 15 min. The synthesized iron particles were separated from the solution by using magnets. The SiO2-nZVI particles formed by this reaction were washed with deionized water to prevent immediate rusting.
2.3. Optimizing Preparation Conditions of SiO2-nZVI
Our study intends to set different preparation conditions to explore the similarities at different conditions of the formed layered structure of SiO2-nZVI particles and examine antioxidant capability and reducing ability of SiO2-nZVI coated particles prepared in different conditions.
Effects of ethanol/distilled water volume ratio: The SiO2-nZVI prepared in various liquid systems containing different ethanol/distilled water volume ratio (1:2, 1:1, 2:1 and 3:1).
Effects of PEG: We explored whether PEG was added to the SiO2-nZVI preparation system.
Effects of TEOS dosage: Various dosages (0.3 - 1.5 ml) of TEOS were added to the reaction mixture.
Effect of TEOS hydrolysis time: The reaction mixture was reacted at various TEOS hydrolysis time (0.5 - 4 h).
2.4. Decolorization Studies
The SiO2-nZVI nanoparticles which were prepared with different conditions were used in dye decolorization test to evaluate their reducing activity. The SiO2-nZVI nanoparticles were incubated with Malachite green solution (100 mg/l). The samples were taken at different time interval and analyzed for dye degradation. The dye decolorization rate was estimated by measuring the absorbance at the λmax (618 nm) of the dye in a UV-vis spectrophotometer (UV-2800, Unico Instruments Co., Ltd., Shanghai, China). The rate of decolorization was calculated from the difference between initial and final absorbance values.
2.5. Characterizations of SiO2-nZVI
TEM was used to visually identify the morphology of SiO2-nZVI. TEM was performed at accelerating voltage of 200 kV. The sample was supported by a copper-mesh TEM grid and dried in air at room temperature. The elemental composition was performed by energy dispersive spectrometry (EDS). XRD was used to investigate the material structure of the SiO2-nZVI nanoparticles. The crystallographic structures of these nanoparticles were analyzed by XRD at wavelength of 0.026˚, scan range from 10˚ to 80˚, voltage 40 kV, electric current 40 mA.
3. Results and Discussion
3.1. Influence of Ethanol/Distilled Water Volume Ratio in the SiO2-nZVI Preparation Process
The effects of the ethanol/H2O volume ratio used in the synthesis of the SiO2-nZVI on the degradation of Malachite green were examined. Table 1 showed the decolorization rate of Malachite green by SiO2-nZVI at various ethanol/distilled water volume ratio. The higher decolorazation rate was obtained at volume ratio 2:1 and 3:1. After 25 min incubation, the decolorization rate was above 99%. The lower decolorization rate was observed at volume ratio 1:2 and 1:1, about 92% Malachite green was decolorized within 25 min.
Our study indicated that a moderate ethanol/distilled water volume ratio improved the decolorization ability of SiO2-nZVI. It was reported that SiO2-nZVI nanoparticles tend to agglomerate rapidly and form larger aggregates in water, thereby losing their reactivity [13] [14] . In the ethanol/distilled water system, adsorbed water molecules on the surface of the generated nanoparticles are replaced by ethanol. Thus, the SiO2-nZVI particles obtained lower surface tension and surface energy, so it can effectively reduce agglomeration force of nanoparticles.
3.2. Influence of PEG in the SiO2-nZVI Preparation Process
PEG is a dispersing agent and acts as a surfactant by adsorption at solid-liquid interfaces; forming a layer of molecules in a membrane prevents particles from being in contact with each other, reduces the surface tension and capillary adsorption capacity, and suppresses crystal growth, resulting in a small size distribution of the nanoparticles [15] . Based on these, the effects of PEG used in the synthesis of the SiO2-nZVI on the degradation of Malachite green were examined in this study, and the result was shown in Table 2. After reaction for 15 min, the removal percentage of Malachite green increased to 99.9% for SiO2-nZVI synthesized in the presence of PEG and to 90.7% for
![]()
Table 1. Effects of ethanol/H2O volume ratio in synthesis procedure on Malachite green degradation.
![]()
Table 2. Effects of presence and absence of PEG in synthesis procedure on Malachite green degradation.
those synthesized in the absence of PEG. These results showed that the nanoparticles’ reducing ability was related to PEG.
3.3. Influence of TEOS Dosage in the SiO2-nZVI Preparation Process
Table 3 showed the effects of TEOS dosage in the synthesis procedure of SiO2-nZVI on Malachite green decolorization. When the dosage of TEOS was 0.5 ml, the azo dye decolorization rate was the highest. The increase in TEOS dosage in SiO2-nZVI synthesis system leads to a decrease in the decolorization ability of the nanoparticles. Just half of the dye was degraded by SiO2-nZVI when the dosage of TEOS was 1.5 ml. With increasing TEOS content, a tight mesh structure was no longer formed, so oxygen would migrate to the particle surface, form an oxidation film, and decrease the availability of reactive nanoparticles, resulting in a reduction in the removal rate of dye water [12] .
3.4. Influence of Hydrolysis Time in the SiO2-nZVI Preparation Process
As shown in Table 4, the influence of coating time in the synthesis procedure on Malachite green degradation were evaluated with increasing reaction time from 0.5 to 4 h. The results showed that the optimum TEOS hydrolysis time for the SiO2-nZVI preparation was 4 h, at which 100% of Malachite green was degraded within 15 min.
It was reported that the reaction time was the main influence factor on the coating layer structure [16] . TEOS is hydrolyzed under alkaline conditions, after the first ethoxy hydrolysis, the second ethoxy hydrolysis rate would gradually accelerate [17] . If the reaction time is insufficient, TEOS may only complete the first step of the reaction, causing incomplete hydrolysis an imprecise reticular structure, and a pore size too large to decrease antioxidation and the reducing capacity. Therefore, in our study, a coating time of 4 h gave SiO2-nZVI with the best reducing and antioxidation capacities.
3.5. Characterizations of SiO2-nZVI
Representative TEM images of SiO2-nZVI were shown in Figure 1. It could be seen that the SiO2-nZVI particles size was about 30 nm. Moreover, from the TEM images, we could clearly see the nanoparticles coated completely with a transparent film. The SiO2-nZVI was spherical and formed linear chains in space, and the surfaces of the coated particles were smooth and uniform. In addition, SiO2-nZVI did not form large aggregates. The chemical composition of SiO2-nZVI was also determined by EDS. The elemental composition of SiO2-nZVI particles were analyzed by EDS, and the spectra confirmed the presence of Fe and Si in the Core-shell SiO2-coated iron nanoparticles.
![]()
Table 3. Effects of TEOS dosage in synthesis procedure on Malachite green degradation.
![]()
Table 4. Effects of hydrolysis time in synthesis procedure on Malachite green degradation.
![]()
Figure 1. Transmission Electron Microscopy (TEM) image of SiO2-coated iron nanoparticles (SiO2-nZVI) and Energy Dispersive Spectromete (EDS) image of SiO2-coated iron nanoparticles (SiO2-nZVI).
Figure 2 shows the results of XRD analysed freshly synthesized nZVI, and freshly synthesized SiO2-nZVI samples. The diffraction peaks of freshly prepared nZVI located at 2θ = 44˚, 64˚ and 83˚ respectively. The diffraction peak of 44˚ reflected that zero- valent iron existed in sample. The other two diffraction peaks indicated that the sample contained iron oxides. The diffraction peaks of freshly prepared SiO2-nZVI were located at 2θ = 27˚ and 44˚. The diffraction peaks from Si (2θ = 27˚) were found in sample. These results indicated that the nZVI particles and SiO2 formed composite by the optimizing synthesis methods.
4. Conclusion
In this study, we have gained surface modified SiO2-nZVI particles with superior
![]()
Figure 2. Powder X-ray Diffraction (XRD) spectra of nanoscale zero-valent iron (nZVI), SiO2- coated iron nanoparticles (SiO2-nZVI).
reactivity and uniform core/shell structure when the particles synthesized with ethanol/H2O ratio of 2:1, PEG of 0.15 ml, TEOS of 0.5 ml. By using the optimized method, the whole SiO2-nZVI synthesis process could be completed within 5 h. Moreover, the optimizing particles had high reductive ability. They could degrade almost 100% of Malachite green (100 mg/l) within 30 min. The TEM results proved that SiO2 film coated full nZVI particles and formed linear chains in space. All these results showed that the new SiO2-based membrane composite material has potential to serve as an effective reductive system for the wastewater treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (J1210053), the Fundamental Research Funds for the Central Universities (2572015- BA04).