A-Site Effect on the Conversion of Bio-Ethanol into Isobutene over Ternary A1ZnyZrzOn Catalysts ()
1. Introduction
Conversions from sustainable biomass-based raw materials to valuable fuels and chemicals have drawn intensive interest to meet grand challenges in global warming and depletion of fossil feedstocks [1] [2] . With the development of biological fermentation, bio-ethanol has become the major product from biomass conversion [3] [4] . Currently, bio-ethanol conversions to highly valuable chemicals are mainly focused on ethanol dehydration to ethylene over solid acid catalysts, such as alumina [5] , zeolites [6] and heteropoly acids [7] or ethanol dehydrogenation to acetaldehyde and then to acetone viaaldol-condensation routes [8] . In particular, bio-ethanol conversion to lighter olefins has become a strategic reaction for the production of highly valuable chemicals. At present, isobutene is predominantly produced via extractive distillation of the C4 fraction from catalytic steam cracking of fossil-based naphtha in ethylene production. With the switching to shale gas for industrial production of ethylene, it calls for alternative methods of C4 light olefins synthesis. Therefore, it is highly desirable to explore new routes to produce isobutene from renewable resources.
Tago et al. [9] found that alkali metal ion-exchanged BEA zeolites exhibited the high yield (55%) of acetone conversion to isobutene as a result of elimination of the strong acidic sites by introduced alkali metal ions (Na, K, Rb and Cs) and inhibition of the formation of aromatics and coke. Wang and co-workers reported the first evidence of direct conversion of bio-ethanol to isobutene (ETIB) over ZnxZryOz mixed oxide catalyst [10] . They rationalized that the balanced acid-base sites of ZnxZryOz were responsible for the direct and high-yield conversion of ETIB. The direct one-step conversion of ethanol to value-added C4 light olefins has long been the target of catalysis research [11] . SiO2-MgO mixed oxides have been found to be active for one-step conversion of ethanol to 1,3-butadiene, and with various dopants introduced, such as Cu [12] , Ag [13] , and other transition metal (oxide)s [14] . The catalytic properties of the binary SiO2-MgO system can be enhanced by promoting the dehydrogenation of ethanol and by shifting the rate-relevant step of the reaction network.
In this work, different A-site metals such as Fe, Cr, and Ni were introduced into binary Zn1Zr8On forming ternary A1Zn1Zr8Oncomposite catalysts, aiming to enhance the catalytic activity for ETIB reaction. Our results show that by introducing Cr, the selectivity of isobutene formation over ternary Cr1Zn1Zr8On mixed oxide composite is markedly increased compared to that of binary Zn1Zr8On. Meanwhile, the contents of zinc and zirconium species were found to be essential for the isobutene production over ternary Cr1ZnyZrzOn.
2. Experimental
2.1. Preparation of Ternary A1ZnyZrzOn Catalysts
To synthesize ternary A1ZnyZrzOn catalysts, we employed the conductive carbon black T100 as the hard template, which was heated at 180˚C overnight before being used. In a typical synthesis, 0.00125 mol of Zn(NO3)2∙6H2O (Sigma-Aldrich, >99.8%), 0.01 mol of ZrO(NO3)2∙xH2O (Sigma-Aldrich, >99.8%), and 0.00125 mol of Ni(NO3)2∙3H2O (Sigma-Aldrich, >99.8%) were dissolved in 40 ml de-ionized water with ultrasonic treatment for 30 mins to obtain a clear and homogeneous solution. Then, 6.0 g of preheated MA100 (Mitsubishi, Japan) was added into the above solution with stirring at the speed of 500 RPM for 2.0 hrs. After that, the mixture was transferred to a ceramic crucible and dried at 110˚C for 12 hrs in oven and further calcined at 400˚C for 4.0 hrs and stay at 550˚C for another 20 hrs to remove the carbon black. The obtained white powders were denoted as Ni1Zn1Zr8On catalysts. When Ni(NO3)2∙3H2O was replaced by Fe(NO3)3∙9H2O, Co(NO3)2∙3H2O or Cr(NO3)3∙9H2O, Fe1Zn1Zr8On, Co1Zn1Zr8On or Cr1Zn1Zr8On composite oxide was obtained, respectively.
2.2. Catalyst Characterizations
Several physical techniques were employed to characterize the structure of synthesized catalysts. a) X-ray powder diffraction patterns (XRD) for all the samples were collected on X-ray diffractometer. b) Morphology and size of Cr1Zn1Zr8On catalyst were probed by field emission scanning electron microscopy (FESEM, Hitachi S4800). c) Nitrogen adsorption-desorption isotherms were recorded on a Micromeritics ASAP 2460 at 77 K, where the specific surface areas (SBET), pore volume (VP) and pore diameter (DP) were calculated by applying the Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) models to the desorption branches.
2.3. Catalytic Activity Measurements
The vapor-phase ETIB reactions were evaluated in a tubular quartz reactor (i.d. 5.0 mm). In each run, 200 mg catalyst pelletized catalyst sample (size range: 40 - 60 mesh) was placed at the center of the reactor tube between two layers of quartz wool and were pretreated in N2 (50 ml/min) at 400˚C for 0.5 h. A mixture of ethanol/H2O (steam/carbon ratio = 5.0) was then introduced into the evaporator (200˚C) by a syringe pump and directly carried into the reaction system by flowing carrier gas of N2. An online Shimadzu 2014 Gas Chromatography (GC) with three analyzing channels was used for full product analysis. The carbon balance was checked in each run and found to be higher than 95% in every case. The ethanol conversion and product selectivity were calculated on a per carbon basis and defined by two following equations:
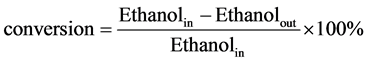 (1)
(1)
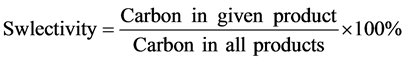 (2)
(2)
3. Results and Discussions
3.1. Catalyst Characterization
3.1.1. XRD
Figure 1 displays XRD profiles of ternary A1Zn1Zr8On composite oxides in the comparison with binary Zn1Zr8On. Tetragonal phase of ZrO2 (PDF#50-1089) are observed for all samples. But the details of the diffraction peaks change greatly with different A-site dopants. Binary Zn1Zr8On only shows diffraction peaks from tetragonal phase of ZrO2. Sharper diffraction peaks of tetragonal ZrO2 are found over Ni1Zn1Zr8On, and some diffraction peaks assignable to NiO (PDF#2901) are obvious. The introduction of Fe did not affect the diffraction peaks of tetragonal ZrO2. Co1Zn1Zr8On shows the weak diffraction peaks of CoO species (PDF#2902) and the peaks belonging to tetragonal ZrO2 remained unchanged. Although additional diffraction peaks are not found after introducing Cr, it is noted that diffraction peaks of tetragonal ZrO2 are weakened. So, when binary Zn1Zr8On composite oxides dope with a little of transition metal species, the changes depend on dopants. The introduction of Fe and Co species cannot affect the crystallinity. An opposite effection of the crystallinity is found when Ni and Cr are added. When Ni and Co are introduced, highly dispersed Ni and Co species were not formed. In this case, the bulk phase of NiO and CoO was formed in there two catalysts.
3.1.2. N2 Adsorption-Desorption
Figure 2 shows that all the catalysts display the type IV N2 absorption-desorption isotherms with H3 hysteresis loops at the relative pressure (P/P0) ranging from 0.80 to 1.0, an indicative of a typical mesoporous structure. This indicates that the porous structure is not destroyed by doping. We also have calculated BET surface area (SBET), pore volume (VP) and pore diameter (DP) from N2 absorption-desorption isotherms using BET and BJH models, and the results are shown in Table 1. It can be seen that only introducing Cr improves the SBET while other dopants introduced into the binary Zn1Zr8On will cause the decrease of specific surface area.
3.1.3. FESEM
Figure 3 presents the FESEM chemical mapping image, which definitely shows the homogeneous distribution
![]()
Figure 1. XRD patterns of A1Zn1Zr8On and Zn1Zr8On catalysts.
![]()
Figure 2. N2 adsorption-desorption isotherms of A1Zn1Zr8On and Zn1Zr8On catalysts.
![]()
Table 1. Physical and chemical properties of A1Zn1Zr8On catalysts.
![]()
Figure 3. FESEM images corresponding element mapping of Cr1Zn1Zr8On catalyst.
of Cr and Zn atoms with Zr and O atoms as a background, which support XRD results that the synthesized mixed metal oxides contain no individual Cr2O3 or ZnO phase.
3.2. Catalytic Performances
Figure 4 presents the product distributions of ETIB reaction over ternary A1Zn1Zr8On and binary Zn1Zr8On catalysts at 500˚C. One can see that the selectivity of isobutene is 37 % and 39% CO2 is gained on binary Zn1Zr8On catalysts. AsNi and Co is introduced forming Ni1Zn1Zr8On and Co1Zn1Zr8On, respectively. There are no notable changes of product distribution with respect to binary Zn1Zr8On. But only acetone selectivity decreases or increases. When Fe is introduced forming Fe1Zn1Zr8On, isobutene selectivity (8%) decreased dramatically which is far below binary Zn1Zr8On. In this case a lot of CO2 (62%) is generated, suggesting that the Fe species favors hydrogen production via steam reforming of ethanol (CH3CH2OH + H2O → CO2 + H2). A large amount of H2 obtained in product effluents also supports this argument. In all ternary catalysts, Cr1Zn1Zr8On shows the best isobutene selectivity (51%), while the acetone selectivity increases to 12% and CO2, CH4 and ethylene selectivity decreases to 30%, 0.7% and 0.9% respectively, suggesting that ethanol dehydration and cracking are suppressed.
Reaction condition: catalyst weight m = 0.20 g; S/C = 5; xethanol = 2.73 mol% (N2 balance); W/F = 0.0975 g∙s∙ml−1, P = 1 atm., T = 500˚C.
As can be seen from Figure 5, the content of ZnO notably affected the product distribution of bioethanol conversion at 500˚C. When y value varies from 0 to 1, ethylene selectivity decreases and isobutene selectivity increases greatly. At the same time, acetone selectivity increases firstly and then decreases. At y = 2, isobutene selectivity decreases and ethylene shows up again. This suggests that the appropriate content of the zinc addition is very important to the good isobutene selectivity.
From above, we can conclude that the functionalized active sites associated with A, Zn and Zr content in A1ZnyZrzOn catalyst should be well matched to obtain the highest isobutene selectivity in ETIB reaction. Although the first step, ethanol dehydrogenation is not a difficult reaction, there is still the intense competition between ethanol dehydrogenation and dehydration. The introduction of transition metal species such as Cr can significantly promote the dehydrogenation and suppress the parallel competitive dehydration. The next steps (acetaldehyde-to-acetone and acetone-to-isobutene) need a proper balance between acid and basic sites, which have been evidenced by appropriate Zn addition. Because of the presence of single oxide, which is badly cooperated with other active sites, the introduction of Co or Ni cannot gain better isobutene selectivity. Especially, the introduction of Fe breaks the reaction path and leads to more by-products. Since ZnO is amphoteric oxide, and the excess or the insufficient of its addition will lead to other side reactions and cannot obtain good isobutene selectivity.
![]()
Figure 4. Product distribution of bio-ethanol conversion over A1Zn1Zr8On and Zn1Zr8On catalysts. Reaction condition: catalyst weight m = 0.20 g; S/C = 5; xethanol = 2.73 mol % (N2 balance); W/F = 0.0975 g∙s∙ml−1, P = 1 atm., T = 500˚C.
![]()
Figure 5. Product distribution of bioethanol conversion over Cr1ZnyZr8On catalysts. Reaction condition: catalyst weight m = 0.20 g; S/C = 5; xethanol = 2.73 mol % (N2 balance); W/F = 0.0975 g∙s∙ml−1, P = 1 atm., T = 500˚C.
4. Conclusion
A series of ternaryA1ZnyZrzOn catalysts using hard template method were synthesized and investigated for the activity of direct ETIB. Introduction of transition metal species into A-site of ZnyZrzOn significantly affected the catalytic reactivity. In particular, introduction of Cr species into Zn1Zr8On is essential for promoting ETIB activity with respect to binary Zn1Zr8On which is closely related to excellent ethanol dehydrogenation capability caused by its redox properties. Meanwhile, appropriate addition of zinc on the catalyst surface is also essential for good isobutene yield. On the whole, the species and the quantity of active sites are the key to the target product isobutene.
Acknowledgements
This work is supported by The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, National Natural Science Foundation of China (21503133), The Scientific Research Foundation for the Returned Overseas Chinese Scholars from State Education Ministry, Natural Science Foundation of Shanghai City (15ZR1419100), and Internal funds from Shanghai University of Engineering Science.
NOTES
![]()
*Corresponding author.