Study of Process Affected by Electrolyte Concentration through Microarc Oxidation on the TC4 Alloy Surface ()
Subject Areas: Biological Materials, Composite Material, Material Experiment

1. Introduction
As a kind of typical biological activity, material calcium phosphate ceramic has the similar chemical composition and structure with the hard tissue of human body, and is the main inorganic component of human bone and teeth. When implanted in the human body, the kind of material can not only lead to bone-formation, but also form synostosis with new bone. With the muscle, ligament or subcutaneous tissue cultivation calcium phosphate ceramic can closely integrate with the organization, at the same time it is no inflammation or irritation reaction [1] - [4] . Therefore, calcium phosphate ceramic material is often used for artificial bone, artificial joint, artificial tooth and bone filling materials, and it is also used in clinical. Surface modification of biological coating on Titanium alloy is included in electroplating, spraying film and high temperature synthesis, but different level of the film and substrate adhesion is poor shortcoming. So the stripping phenomenon is inevitable after implanted, which leads to implant failure or a shorter implant life.
MAO is an economic, efficient and environmentally friendly technology for surface treatment on lightweight metals (aluminum, magnesium, zirconium, titanium, etc.) or metal alloys [5] - [8] . Many properties of MAO coating including bonding strength with the substrate, thickness of film, micro hardness, anti-wear and corrosion resistance are all relatively excellent [9] - [12] . But the coating outside in general is a loose layer, while the internal one is a dense layer. Usually related to dense layer, the loose layer is thick and loose organization, and mainly consists of anatase phase with relatively low hardness for the Ti alloy MAO coating [13] - [16] .
But in the micro arc oxidation process for preparing biocoating of on titanium alloy surface, the composition of formed coating is greatly affected by the electrolyte concentration, while the electrolyte concentration is mainly decided by the content of calcium and phosphorus in chemical reagent solution. When the electrolyte concentration wants to obtain one coating with a certain calcium/phosphorus ratio relationship, the chemical reagent allocation becomes the key factor restricting the success of the experiment. So, we must research the relationship between concentration of solution and coating component of calcium and phosphorus.
2. Experimental Details
2.1. Experimental Material
Experiment treated substrate material is TC4 titanium alloy (mass fraction of elements, such as Table 1).
TC4 alloy plates were used as specimens whose measurement is 80 mm × 40 mm × 3.5 mm. The plates were ground with 350, 700 and 1000 # abrasive papers, flushed with acetone and distilled water for 20 min, and dried at 40˚C.
2.2. Prepared Electrolyte
Prepared electrolyte is as shown in Table 2. The concentration of 10% ammonia water was used to regulated pH = 11 - 12.
2.3. Micro Arc Oxidation Equipment and Electrical Parameters
The 20 KW DC/AC micro arc oxidation device of the laboratory developed was used to treat the specimens, the equipment is composed of pulse power supply, electrolytic tank, mixing system and cooling system. The positive pole of the power supply was connected with the specimen clamped by special and as anode, then stainless steel was as cathode electrolytic tank.
The applied voltages (effective values of voltage) of 510 V were used to prepare MAO coatings. The power waveform, frequency and duty cycle were unipolar pulse, 500 Hz and 20%, respectively; it was one specimen for one prepared solution, and the number was NO. 1 - NO. 12.
2.4. Analysis Equipment
JEOL-JSM-5900LV scanning electron microscope (SEM) was used to observe coating morphology and structure. The electronic energy spectrum analyzer (EDS) was used to analyse the Ca and P element content in the coating; The universal meter was used to measure the electrolyte voltage; The friction tests of the film were carried out by friction wear testing machine with the type of MMW-1; JA type Tailong electronic balance was used to weigh wear, and its accuracy is 0.001 g.
3. Result and Discussion
3.1. Effect of Solution Concentration on Biocoating Morphology
In the electrolyte molar ratio of Ca and P as to 1.6, surface and section morphology of micro arc oxidation coatings prepared with different concentration are shown in Figure 1 and Figure 2. The figure shows the coating surface pore is become larger with electrolyte concentration increased, and coating is become coarse, as well as dense layer thickness increased.
3.2. Effect of Solution Concentration on Biocoating Composition
Micro arc oxidation test solution is mainly composed of Na+, Ca2+, OH−, 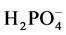 , NH4+, CH3COO−, Ca(H2PO4)2, Ca(OH)2, and Ca3(PO4)2 particles. The Ca(OH)2 and Ca3(PO4)2 is insoluble in water, but the colloidal particles with negative charge can be formed with negatively in water, in addition calcium and phosphorus element can exist by Ca2+ and
, NH4+, CH3COO−, Ca(H2PO4)2, Ca(OH)2, and Ca3(PO4)2 particles. The Ca(OH)2 and Ca3(PO4)2 is insoluble in water, but the colloidal particles with negative charge can be formed with negatively in water, in addition calcium and phosphorus element can exist by Ca2+ and  formation in electrolyte.
formation in electrolyte.
Figure 3(a) and Figure 3(b) respectively show the changes of calcium and phosphorus content of biocoating with different electrolyte concentration. In Figure 3 it is can be seen with the same electrolyte concentration, if solution molar ratio of Ca/P in electrolyte is lower, on the contrary, it is higher for the calcium and phosphorus content in the coating. But for the electrolyte of molar ratio of Ca/P determined, with the concentration improvement of (CH3COO)2Ca and NH4H2PO4, in biocoating the calcium and phosphorus content is not increased but decreased, as well as for the higher concentration concentration, in the coating the calcium content is decreased slowly, while the content of phosphorus in the whole concentration range is rapid decline, and the rate of decline is significantly greater than that of calcium, especially for the lower molar ratio Ca/P in electrolyte.
The main reasons of these phenomena are as follows, with electrolyte solution concentration increased, more charged particles of calcium and phosphorus are produced, which is contribute to increase the conductive performance of the electrolyte, and the equivalent resistance of the electrolyte is decreases, who lead to the pressure drop reduced, as shown in Figure 4. When the electrolyte concentration is increased from 8 g/L to 32 g/L, at the same time in the electrolyte the content of the colloidal particles of Ca(OH)2 and Ca3(PO4)2 is improved, and these colloidal particles play an impedance function, which could make the electrolyte pressure improved. The solution, biocoating and titanium alloy substrate is roughly equivalent circuit of series state, so when in the micro arc oxidation stability stage, it will make the pressure drop in micro arc discharge channels decreased, who
![]()
![]() (a) (b)
(a) (b)
Figure 3. Effect of electrolyte concentration on biocoating: (a) Effect of Ca content; (b) Effect of P content.
![]()
Figure 4. Effect of electrolyte concentration on voltage.
directly affects the gas discharge breakdown state, and indirectly affects the calcium and phosphate ions number into the layer and combined to form calcium phosphate salt opportunity, which leads calcium and phosphorus content in the coating is decreased. But when the electrolyte concentration is increased to a certain extent, and colloidal particles concentration is close to saturation, the voltage drop of electrolyte is tend to the steady state, so the calcium and phosphorus content in the coating is changed slowly. Because the phosphorus than calcium ion is more difficult to get into discharge channel [12] , at the same time with the same electrolyte concentration, Compared with the calcium ion concentration is increased, the phosphorus ion content is on the decline, so in the coating the phosphorus ion content is decreased significantly. So in the same concentration, in the electrolyte of lower Ca/P molar ratio the higher phosphorus content should be guaranteed, which will ensure that there are more P ions to get into the discharge channel, and there is more calcium and phosphorus is contained in the coating.
It is also seen in Figure 3, with the increase of the electrolyte concentration, although calcium and phosphorus content is increased, it cannot guarantee that the calcium and phosphorus content in the coating is increased at the same time, on the contrary, it is decreased. So the configured electrolyte concentration is too should not be too large, and the same for molar ratio of Ca/P in electrolyte. But because of the calcium in addition to is participated to be prepared the biocoating, also is fabricated other substances in electrolyte, so as to make the calcium content is relatively surplus in the electrolyte.
3.3. Relationship of Ca/P Ratio in Coating and Electrolyte Concentration
Although calcium and phosphorus compounds concentration is changed compared to the same ratio in electrolyte, in the biocoating calcium and phosphorus content change is not the same trend, which causes that although Ca/P molar ratio is consistently kept, the mole ratio in the biological coating is in change. Following to electrolyte concentration of calcium and phosphorus compounds is increased, as well as the Ca/P molar ratio in biological coating is the same trend, and in the electrolyte of Ca/P molar ratio with bigger value it is more significant changes, as shown in Figure 5. In the electrolyte of Ca/P molar ratio values with 1.2, 1.6 and 2, Ca/P molar ratio of hydroxyapatite (1.67) is as a sign of biocoating Ca/P molar ratio, if wants to obtain the biological coating of ratio of 1.67, the MAO experiment concentration of the electrolyte are about 26.4, 16.2 and 14.5 g/L, respectively, as shown in Figure 5 with “*” correspond to the abscissa values shown.
This conclusion can guide the configuration of electrolyte in the process of micro arc oxidation, and then determine reasonably proportioning of the calcium and phosphorus compound reagent.
3.4. Effect of Solution Concentration on the Wear Resistance of Biocoating
From the wear capacity in Figure 6 and wear time curve, it can be seen that for the electrolyte concentration of 16 g/L and 24 g/L, the former wear capacity (in 30 min) are also less than 8 g/L, but the concentration of electrolyte is 32 g/L, it is the larger amount of wear and tear at the same time. That is mainly due to the more thick
![]()
Figure 5. Relationship of Ca and P of biocoating and electrolyte concentration.
![]()
Figure 6. Relationship of wear time and wear weight loss of the MAO coatings at different electrolyte concentration of 1.6 mole ratio of Ca and P.
loose layer of biocoating which is prepared in high electrolyte concentration condition, so the wear resistance is poor, and early wear mainly is occurred in the loose layer, ultimately, the wear capacity is larger. To the lower concentration electrolyte level (8 g/L), because of the lower concentration the process of growth of the prepared coating is not sufficient, which does not meet the minimum requirements of micro arc oxidation process for electrolyte concentration. So it is not sufficient for micro arc oxidation discharge in the parts of micro area, and the plasma discharge fully is not unrealized, the prepared coating is mainly in the loose layer with relatively thicker, who affected the effective generation of dense layer, so the wear amount is large in the early stage. This shows that proper concentration is helpful to improve the surface properties of the biocoating, and the coating surface is smooth and dense, as shown in Figure 2. In the later period of wear because of contact of the friction pair and the dense layer (In the early of outer basic is nearly worn off), and because the biological membrane pores can store lubricating oil to improve the lubrication effect, and later the wear volume is gradually reduced and finally tends to zero.
4. Conclusions
1) The effect of calcium and phosphorus molar content in the biocoating with electrolyte concentration is studied. With the electrolyte concentration increased, the calcium content and phosphorus content in the coating are all decreased, while the change trend of the content of phosphorus is more obvious;
2) The effect of calcium and phosphorus molar ratio in the biocoating with electrolyte concentration is studied too. With electrolyte concentration increased, the molar ratio of calcium and phosphorus in the coating is increased at the same time;
3) The relationship between biological membrane calcium to phosphorus ratio and electrolyte concentration is researched. If we want to prepare a biocoating with one calcium phosphorus ratio, different electrolyte concentration of Ca/P ratio can be confirmed by it, and reagent ratio of calcium and phosphorus compounds can be determined approximately;
4) The too high or too low electrolyte concentration will all affect wear resistance of the biocoating, but the suitable amount of electrolyte concentrations contributes to improvement of surface properties of the coating.
NOTES
![]()
*Corresponding author.