Preparation of Composites by Nitro Aluminothermic Processes, over β–SiAlON Matrix in the SiAlON-SiC-Al2O3 System ()
Received 31 March 2016; accepted 12 June 2016; published 15 June 2016

1. Introduction
In recent years, SiAlON ceramic is intensely used for fabrication of wear resistant, abrasive, construction designation objects since it is characterized by unique mechanical properties, thermal resistance and simultaneously resistance to the impact of chemical medium at high temperatures. This enables us to call SiAlONs “smart super ceramics”.
SiAlONs are used in various spheres of technology. Irrespective of a great number of works of researchers of many countries [1] - [9] dedicated to preparation of SiAlONs, methods of SiALONs synthesis offered in those papers, mostly used in laboratory experiments, there is rather scarce information about methods of their industrial preparation. It is connected with high sensitivity of the products of synthesis to chemical composition of raw material and parameters of the processes going on during synthesis which complicate forecasting of phase composition of target products.
With this in view, study of regularities of SiAlON synthesis and selection of reliable methods to receive new functional materials are the urgent task for modern materials science.
SiAlONs are distinguished according to their crystalline structure, silicium nitride, silicium oxynitride, aluminum nitride, mullite structure and diverse Al:Si ratio [10] - [13] . Crystalline structure of SiAlON is similar to the structure of the substance to which the medium of its formation is closer. There are several types of SiAlONs: α; β; X; O1; H; R. Methods of SiAlON synthesis differ by initial material and conditions of the procedure (temperature, duration, pressure, atmosphere composition). General formula of β-SiALON is: Si6−X AlxOxN8−X (where x= 0 ÷ 4.2).
It is considered that SiAlON is a solid solution of Si3N4 with aluminum nitride and oxide [14] - [18] .
The system Si-Al-O-N can be presented as four-component, schematically given tetrahedron, with the constituting elements at vertexes (Figure 1).
Structures and names of SiAlON phases are given in Table 1 [19] - [21] .
![]()
![]()
Figure 1. Versions of schemes of distribution of constituting components in Si-Al-O-N system.
![]()
Table 1. Names and structures of SiAlON phases.
2. Major Part
Composites were prepared via the method of reactive sintering and nitro aluminothermic processes by sintering at 1450˚C in nitrogen medium (Table 2) [22] - [28] .
Initial materials used in the synthesis were: geopolymer, aluminum powder, silicium, silicium carbide, aluminum oxide. To inhibit growth of crystals in the composite and to improve sintering process we used magnesium oxide and oxide of rare earth element?yttrium, as admix. MgO and Y2O3 were added to the powder at 1 and 1.5 mass%, correspondingly. High melting temperature of oxides provides for creation of high temperature eutectic melt in the mixture at its heating, which contributes to material sintering process, which is essential for obtaining objects characterized by high thermal resistance and strength.
To improve the sintering process we added glass perlite and refractory clay in small quantities. We compiled mixes and offer their compositions in Table 2.
A mix was prepared according to the materials and concentrations given in Table 2; specimens were molded into cylinders, with sizes: d―15 mm, h―15 mm, under 20 mPa pressure, by semi-dry method―moisture equaled to 8%. At 110˚C, after drying, specimens were sintered at 1450˚C, under nitrogen medium, with 1 hr standing. A device used for specimen sintering is given in Figure 2, while temperature regime for sintering in Figure 3.
A device for silicium specimens sintering (Figure 2) consists of a furnace (1), silicium carbide heaters, mark TK 30/200. Nitrogen is supplied to the furnace from the bottle (2) through rubber pipe, with a tap on it (3). At the entrance of the furnace velocity of nitrogen coming from the bottle (4) through choke is regulated .Then nitrogen enters refractory corundum pipe (5), which is hermetically closed from both ends. From one end the pipe is supplied with nitrogen and at the exit of the second end, there is a choke with water (6). It regulates pressure in the furnace so that the velocity of nitrogen leaving the pipe was lower than the velocity of nitrogen that enters the reaction pipe. Then nitrogen is released to atmosphere. Furnace temperature is regulated by a transformer (7) and a voltmeter (8) connected to it, while temperature is measured by means of a millivoltmeter (9) and platinum-rhodium thermocouple (10). Rate of increase of temperature is 250˚C/hour. Pressure in nitrogen bottle is regulated by a reducer, which is fixed at the nitrogen bottle.
Physical-technical properties of the obtained specimens have been investigated and are given in Table 3.
To receive the material to be characterized with high exploitation properties we selected the composite CH-6. Specimens sintered at 1450˚C were ground and prepared for hot compaction. Precursor was prepared in a thermostat at 150˚C and was cold pressed twice at 15 and 25 mPa; it was hot pressed at 1600˚C under 30 mPa, Vacuum equaled to 10−3 Pa, standing at final temperature?10 - 12 min.; sintering regime was: from 20˚C to 500˚C, 7˚C/min, from 500 to 1400˚C, 5˚C/min, from 1400˚C to 1600˚C, 10˚C/min; cooling 10˚C/min. Temperature regime of sintering is offered on Figure 4.
We have investigated physical-technical indices of specimens hot-compacted at 1600˚C. The results are offered in Table 4. Open porosity of hot-compacted specimens equals to 0.5%, while limit of strength at compaction―1940 MPa; hardness―1140. Considering the data given in the Table we can conclude that 1600˚C is sufficient for complete hardening of specimens.
Table 5 offers micromechanical characteristics of hot-compacted specimens.
![]()
Figure 2. A device designed for sintering via nitro aluminothermic processes created by us at the Bionanoceramic and Nanocomposite Materials Science Center of Georgian Technical University.
![]()
Figure 3. Temperature regime for sintering of the obtained specimens by the method of reactive sintering.
![]()
Figure 4. Temperature module for sintering of hot-compacted specimens.
![]()
Table 3. Properties of the composites obtained by reactive sintering.
![]()
Table 4. Physical-technical indices of hot-compacted CH-6 specimen.
![]()
Table 5. Micromechanical characteristics of hot-compacted specimen CH-6.
Lawn and Marshall [29] [30] have also introduced quantitative brittleness factor (B), which can be obtained experimentally by dividing micro hardness value by the critical stress intensity factor (Kic) of the specimen: B = Hv/Kic. A lower brittleness factor means lower probability of catastrophic crack propagation in a specimen during machining.
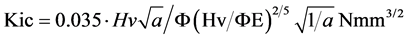
where Φ: form factor; l: crack length; a: averaged half of impression diagonal; E: elasticity module.
For machining of materials n-factor is an important parameter [31] . This factor enables us to speak about easiness of its machining; n = 0.643 - 0.122 HV. It is claimed that a specimen can be easily machined if it has a positive n value and cannot be easily machined if it has a negative n. In case of our material, treatment by cutting as well as machining for grinding is difficult due to high hardness of the material. We have to state that in the process of disk cutting by diamond disk we came across with high resistance, which damaged some diamond saws and it, quite naturally resulted in propagation of cracks in the material. This reduced mechanical properties of the material to a certain degree. It would be better to have the possibility to perform laser cutting treatment. Dynamic microhardness and elasticity modules were determined on dynamic ultra microhardness tester DUH-211S corresponding to the demands of ISO-14577 International Standards, which is generally used for determination of mechanical characteristics of solid bodies surfaces. Results are offered in Table 6 and Table 7.
Table 6 offers results of tests performed on the specimen CH-6 hot-compacted at 1600˚C. Impressions are taken in matrix. Impressions were taken several times, and results are given in the Table, where average hardness HV: 11.60 GPa.
Dynamic microhardness (DH) was determined in the process of testing according to the load force value on the
![]() Test result
Test result ![]()
Table 6. Test condition-CH6-1600(200gr.).
Indenter elastic 1.140e+006 [N/mm2] Indenter Poisson’s ratio 0.070.
![]() Test result
Test result ![]()
Table 7.Test condition-CH6-1600(100 gr).
Indenter elastic 1.140e+006 [N/mm2] Indenter Poisson’s ratio 0.070.
indenter and depth of its penetration into material; its value was computed by the formula: DH=a x F/h2; where a is a constant value and depends on indenter form; for Vickers indenter a = 3.8584.
Advantage of the method compared to the common, static method, that is measuring linear sizes of impression (diagonal) is that it consists of both plastic and elastic components. Results of measurements don’t depend on impression sizes, load and non-homogeneity of flexibility recovery.
Dynamic hardness was determined in loading-unloading regime, by taking seven readings at each concrete loading, by discarding two end values and by averaging the remaining five values. Corresponding value of micro hardness was determined automatically. Hold-time at maximum load equaled to 5 sec., at the end of unloading 3 sec (Figure 5(a) and Figure 5(b)) and (Figure 6(a) and Figure 6(b)).
Indenting was performed in specimen matrix, which consisted of β-SiAlON. Testing has shown that its mean dynamic hardness equals to DHV = 10.29 GPa, which is a rather high value [32] - [34] .
From the load-unload ratio graph (Figure 5) the elasticity module value is determined by defining rigidity S = (dF/dh) h-hmax. It is a tangent of load-unload graph at the starting point of unloading. A device defined flexibility module of the material under the study, which in case of our specimen equals to: E = 199 GPa.
Table 7 offers results of tests performed on the specimen CH-6 hot-pressed at 1600˚C. Impressions are taken in matrix. Impressions were taken several times, and results are given in the Table, where average hardness HV: 11.54 GPa. Elasticity module E = 203 GPa.
Figure 7 shows impression taken in SiAlON matrix, which shows that impression form is clear, with clearly cut edges. Along the edges similar size cracks can be observed which speaks about matrix homogeneity.
Table 8 offers results of testing of SiC grains of the composite CH6-1600 microstructure. Indenting was performed at 199 gr load on grains. Average hardness value HV for silicium carbide was 22.6 gPa; dynamic hardness DH-15.2 GPa, elasticity module E-289 GPa, which are rather high values.
![]() Test result
Test result ![]()
Table 8.Test condition-CH6-1600-(SiC).
Indenter elastic 1.140e+006 [N/mm2] Indenter Poisson’s ratio 0.070.
![]() (a)
(a)![]() (b)
(b)
Figure 6. Load-inload curves for indenter penetration depth into material: (a) load force at indenter; (b) depth of indenter penetration into material.
![]()
![]() (a) (b)
(a) (b)
Figure 7. Impression taken in SiAlON matrix, load (a) 200 gr; (b) 100 gr.
Limits of impressions taken from carbide grains are clear (Figure 8); crack, which was created on the grain as a result of indenter load doesn’t spread beyond the grain limits, matrix inhibits crack spreading and the composite hardness retains its value.
To investigate phase composition of a composite CH-6 obtained via sintering and hot-compaction at 1600˚C we used X-ray and electron microscopy methods.
X-ray analysis was performed on X-ray diffraction apparatus “DRON-3”, roentgenogram was taken at 22.5˚ - 65˚ angle interval, at 2˚ min speed. Both roentgenograms are given in Figure 9 and Figure 10.
Composite X-ray shows diffraction maximums characteristic to SiAlON dhkl: 7.95; 5.63; 3.85; 3.65; 2.520; 2.19; silicium carbide- dhkl: 2.63; 2.370; 2.19; 2.014; corundum-dhkl: 3.49; 2.52; 2.36; 2.090.
As is seen from both X-ray the CH-6 composite consists mainly of β-SiAlON, which has hexagonal structure. Alongside with the β-SiAlON it contains silicium carbide and corundum, which are introduced into the mix from the very beginning. In both cases composite matrix is β-SiAlON and it is hardened by corundum and silicium carbide crystals.
Electron-microscopic studies were performed by JEOL firm electron-raster (scanning) microscope. The method is based on reflection of the reflected electrons and secondary ejected electrons.
To carry out phase analysis of the composite by reflected electrons we broke a specimen parallel to the surface. Fracture must be new. Results are given in Figure 11 at: a) ×270, b) ×1000, c) ×2200, d) ×2700 magnifications.
Figure 11 shows a matrix consisting of β-SiAlON, which confirms the data of X-ray analysis.
Electron microscopy images of CH-6 composite shows the well sintered specimen surface, with clearly observable crystals of the main phases which are in the composites hot-compacted at 1600˚C, in particular as its matrix―SiAlON, SiC and corundum.
Pores are not observed in any specimen. X-ray diffraction analysis data conform to images of electron microscope analysis. It is apparent that at hot-compaction the mineralogical composition of the composite suffers no changes, compared to the specimens obtained by reactive sintering, which becomes more vivid at greater magnification.
X-ray diffraction microanalysis of the same specimen was performed on OXFORD INSTURMENTALS detector X-max, which enabled us to receive general composition of the composite elements. Results of analysis are given in Figure 12.
Figure 12 offers X-ray microanalysis at the spectrum 1 section of CH-6 composite obtained by hot-compac- tion, together with percentage concentration of the constituting elements; it shows that the main component of the composite is SiAlON.
Figure 12 offers percent composition of SiAlON: silicium, aluminum, oxygen and nitrogen. Besides, the element, carbon was fixed there which refers to the presence of SiC. Increased volume of oxygen and presence of aluminum refer to the presence of SiAlON as well as corundum in the composite.
The schemes for element concentrations are offered for relatively short sections spectrum 2, 3, 4, 5 (Figure 13) Schemes mirroring elements concentration at relatively short sections spectrum 2, 3, 4, 5 are offered. X-ray microanalysis of the points given on the image gives clear picture of concentration of elements of phases present
![]()
![]() (a) (b)
(a) (b)
Figure 8. Impression taken from SiC grain at 100 gr. load.
![]()
Figure 9. X-ray of CH-6 composite (1450˚).
namely at those points. The image shows percentage of the constituent elements of the main phases SiAlON, silicium carbide and corundum. At all sections percentage ratio is almost similar, which refers to homogeneous structure of the obtained composite CH-6.
3. Conclusions
CH-6, CH-7 and CH-8 composites were obtained on the base of geopolymer, silicium carbide, aluminum oxide, silicium and aluminum powder by the method of reactive sintering, via nitro aluminothermic processes.
Physical-technical properties and phase composition of the composite were investigated. The main phases are:
![]()
![]()
Figure 12. X-ray microanalysis of CH-6 composite in the section spectrum 1.
β-SiAlON, which is a matrix of the composite, silicium carbide and corundum.
The obtained porous (15.2%) composite was regrinded and the precursor was prepared in a thermostat at 150˚C, by double compression at 25 mPa.
Precursor was compacted hotly, in vacuum, at 1600˚C, under 30 mPa and at standing for 10 - 12 min at final temperature. The obtained CH-6 composite is characterized by high hardness HV-11.60, density 2, 97 g/cm3 and strength at compaction 1940 MPa and strength at bending −490 MPa [35] . Mineralogical composition remained unchanged. Materials, according to their properties can be used for making high refractory articles, such as
jackets to secure thermocouples, furnace bedding, cutting tools for metal and wood treatment, in rocket spatial technology and others.