Temporal and Spatial Distribution of Nutrients and HABs at Coastal Water of Kota Belud, Sabah ()
Received 17 March 2016; accepted 23 May 2016; published 26 May 2016

1. Introduction
Harmful Algal Bloom (HAB) is the process of sudden increase in the biomass of cells that can bring negative impact on the aquatic organism living that environment. Pyrodinium bahamense var. compressum, Alexandrium spp, Karlodinium veneficum and Gymnodinium are the most common HABs species in Malaysian water [1] - [3] . Species has specific environmental requirements for the optimal growth. Growth characteristics of HABs are generally limited by the availability of dissolved inorganic nutrients [4] as well as depended on other physical processes [5] . In addition of that, occurrence of harmful algal blooms is strongly influenced by monsoon seasons [6] . Decrease in dissolved inorganic nutreints input from river is identified as major driver of the decreasing level of nutrients in the inshore water affecting algal bloom formation.
The west coast of Sabah in Malaysia experiences annual harmful algal blooming. West coast of Sabah is an important agriculture region. High nutrient runoff from agriculture field results in harmful algal blooms. Because of this reason, harmful algal bloom monitoring programs are concentrated on the west coast of Sabah particularly in Kimanis bay [3] . In general, eleven species of harmful algal bloom species have been identified in both west and east coast of Sabah [1] . Blooming of HABs species is annual event, but the process still not clear. Not enough information is available regarding the blooming of HABs in west coast of Sabah, mainly in Kota Belud area. During the Southwest monsoon (wet season), the concentration of nutrients in the west coast of Sabah is relatively higher due to runoff of artificial fertilizers from huge agriculture crop-land resulted in accumulation of dissolved inorganic nutrients in estuaries and river [7] . This study was attempted to identify the harmful algal species that present in the coastal water of Kota Belud and to determine diversity of species, abundance and distribution pattern in wet and dry seasons. This study is also undertaken to establish the relationship between the cell densities of harmful algal bloom species with dissolves inorganic nutrients.
2. Materials and Methods
2.1. Description of Study Area
Kota Belud is located in the Southeast of South China Sea and Northwest of Sabah (Figure 1). Kota Belud area contains approximately 1200 ha of mangrove swamps, the largest being the Abai River mangroves, which is gazette as reserve mangrove forest. Smaller mangroves areas are also line Bira Biraan River just north of Kuala
![]()
Figure 1. Location of study area with the four transects (A, B, C and D) with eight stations (A1, A2, B1, B2, C1, C2, D1 and D2) for collecting samples during study period.
Abai and Tempasuk River. The mangroves at the mouth of Bira Biraan River have been cleared for aquaculture. Kuala Abai and Kuala Tempasuk are the two outlets of a major river system that drains a large part of the north western part of Mount Kinabalu, discharge relatively large sediment plumes, whereas Kuala Rampayan at the northern part carry less sediments [8] . So, the study area was under dynamic interface mainly river discharge from Abai and Tempasuk river, wave actions and tidal currents from the South China Sea.
Sampling locations of this study consists of four transects where each transect consists of two stations which was located at 0.5 km and 5 km away from the land toward the sea. Station A1 and station A2 in transect A were located at the mouth of Abai River (the largest coverage of mangrove). Station B1 and station B2 in transect B were located at the river mouth of Tempasuk River which carry the largest sediment plumes from Mount Kinabalu. Station C1 and C2 in transect C were located at the river mouth of Bira Biraan which was dominated by agriculture activities. Station D1 and station D2 in transect D were located in the Kuala Rampayan that carry minute sediment. Sampling was done September (dry season) to December (wet season).
2.2. Field Analysis (In-Situ)
Environmental parameters including temperature (˚C), salinity (psu), dissolved oxygen (%), turbidity and pH value in each of stations were taken by using a multi-function environmental sensor.
2.3. Sampling Water for Dissolved Inorganic Nutrients and Determination of Cell Density
Surface seawater from each station was collected to a depth of 0.5 m from surface. One Liter of water sample was filtered through 0.45 µm pore-size membrane filters, and stored frozen until analyzed. Another ten Liter of water sample passed through the phytoplankton net having mesh size of 20 µm and was collected in 500 ml plastic bottle and immediately preserved with Lugol’s solution.
2.4. Sampling Phytoplankton Cells for Species Identification
Phytoplankton was collected by towing plankton net with 20 µm mesh size to a depth intermediate of 0.5 m. Then, the concentrated phytoplankton was transferred to a bottle and preserved with Lugol’s iodine for microscopic analysis in the laboratory.
2.5. Analytical Parameters
The dissolved inorganic nutrients concentration of NO3-N, and PO4-P were analyzed in the laboratory at Borneo Marine Research Institute, University Malaysia Sabah, using the spectrophotometric methods [9] . Species identification was done by referring the approach done by [10] .
In the laboratory cells counting were performed by using Sedwick rafter method and cell density was estimated by using Stirling cell density formula.
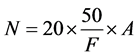
where
N: No. of plankton cells per liter of original water
A: no. of plankton counted
F: total volume (ml) of sample counted
Statistical Analysis
Statistical analyses were performed using the SPSS Windows Statistical Package (Version 19.0). Tests were judged with significant at p < 0.05 level. All variables were tested for normality and homogeneity of variances. Data which satisfy the assumptions of normality and homogeneity, parametric tests (one-way ANOVA, Independent t-test, and Pearson correlation test) were performed.
3. Results
3.1. Spatial and Temporal in Situ Parameters at Kota Belud’s Water
During the dry season, the physical water quality parameters of Kota Belud’s coastal water were relatively constant at all the stations (Table 1). Saturation of dissolved oxygen, value of pH, salinity, temperature and transparency were ranged from 100.9 to 114.7%, 8.13‰ to 8.22‰, 28.9‰ to 29.4‰, 30.3˚C to 30.9˚C and 10.0 to 14.5 m, respectively. No significant difference (p > 0.05) were observed in dissolved oxygen, pH value, salinity, temperature and transparency among all the sampling stations in dry season.
Wider range in the physical water quality parameters were noticed during wet season in the study area. The saturation in dissolved oxygen, value of pH, temperature, salinity and transparency were ranged from 101.1 to 106.5%, 8.22‰ to 8.33‰, 24.4‰ to 29.8‰, 30.0˚C to 30.7˚C and 1.0 to 11.0 m, respectively. There were no significant (p > 0.05) in dissolved oxygen, pH and temperature were recorded among all stations. However, salinity and transparency level at station B1 and B2 were comparatively lower and higher, respectively than salinity recorded from other stations (A1, A2, C1, C2, D1 and D2).
3.2. Spatial and Temporal Nutrients Concentration
The concentrations of NO3-N in study area during dry season were in the range of 0.032 to 0.049 mg/l, but atwet season the values were in the range of 0.038 to 0.070 mg/l (Figure 2). No spatial significant difference (p > 0.05) in nitrate concentration was observed at dry season in spatial distribution. However, the concentration of 0.030 ±
![]()
Figure 2. Concentration of Nitrate-nitrogen (mean ± SD) for each stations during dry and wet season.
![]()
Table 1. Values of in situ parameters (saturation in dissolved oxygen, pH, salinity, temperature, and transparency) collection from the study area during dry season and wet season.
0.003 mg/l nitrate at station B1 was significantly higher (p < 0.05) than the concentration obtained at station B2 compared to other stations at the wet season. Comparatively the higher concentrations of NO3-N were observed in all station during wet season (Figure 2).
Concentration of phosphate in Kota Belud’s coastal water was ranged from 0.007 to 0.008 mg/l and 0.007 to 0.030 mg/l during dry season and wet season, respectively (Figure 3). No significant difference (p > 0.05) was observed in spatial distribution of phosphate concentration among all the stations. During wet season, the phosphate concentration at stations that were located near the shore line in coast (station A1, B1, C1 and D1) water were relatively higher than the phosphate concentration at station located 5 km away from the coast (A2, B2, C2 and D2). The phosphate concentration in station A1, B1 and C1 were significantly higher (p < 0.05) than other stations where the highest Concentration of phosphate of 0.070 ± 0.008 mg/l was recorded from station B1 during wet season.
3.3. Harmful Algal Bloom Community at Coastal Water of Kota Belud
A total of nine harmful algal bloom species were identified at coastal water of Kota Belud. Six species (Pyrodinium bahamense, Prorocentum micans, Neoceratium furca, Prorocentum sigmoides, Dinophysis caudate, and Neoceratium fursus) belongs to Dinophyceae and three species (Thalassionema nitzchoioides, Chatoceros affinis, and Rhizosolenia sp.) belong to Bacillariophyceae. The most dominated species was Chatoceros affinis (80.61% of the total count of 24.04 × 104 cells/l) which showed the highest percentage of cell density during both at dry and wet season in the study area. The second dominated species was Thalassionema nitzchoioides constituted of 12.76% of the total count of 24.04 × 104 cells/l. (Table 2).
![]()
Figure 3. Concentration of phosphate-phophorus (mean ± SD) for each stations during dry and wet season.
![]()
Table 2. Harmful algal blooms species with cell density (×104 cells/l) in Kota Belud’s coastal water during dry and wet seasons.
3.4. Spatial and Temporal Distribution of HABs
The highest harmful algal blooms cell density of (5.01 ± 0.50 × 104cells/l) was observed at wet season in station A1. This value was significantly higher (p < 0.05) than the cell density of HABs species from wet season from other stations, except station B1 and B2. However, HABs cell density in transect A (A1 and A2), C (C1 and C2) and D (D1 and D2) were significantly higher (p < 0.05) at wet season compared to the cell density of the HABs species collected at dry season.
3.5. Influence of Nutrients Concentration on the Harmful Algal Blooms Cell Density
The HABs density was strongly influenced by nutrient concentration where increase in nutrients (nitrate and phosphate) concentration associated with increase in HABs cells density. Nitrate concentration showed a strong positive corellation with HABs density (R2 = 0.802), while a weak positive correlation between phosphate and HABs density (R2 = 0.514) was observed (Figures 4-6).
![]()
Figure 4. Total cell density of harmful algal blooms species for each stations during dry and wet season in Kota Belud coastal water.
![]()
Figure 5. Relationship between nitrate-nitrogen concentration and harmful algal blooms cells density in Kota Belud coastal water.
![]()
Figure 6. Relationship between phosphate-phosrous concentration and harmful algal bloomsin Kota Belud coastal water.
4. Discussion
4.1. Spatial and Temporal Environmental Parameters at Kota Belud’s Water
Current study shows no significant spatial differences in temperature, Secchi depth, pH, Secchi depth (visibility), salinity and dissolved oxygen observed among all 8 stations at dry season. This might be must be due to a small area covered and lack of sample frequency, which was only the limitation of this study. However, spatial differences in salinity and visibility were recorded in station B1 and B2 during wet season. Surface runoff of sediment and discharge of river directly causes the lower visibility in water and the lowest salinity Average rainfall at Kota Belud during dry season was 280 mm while average rainfall during wet season was 400 mm [11] . Both of the Station B1 and B2 located right in front of Tempasuk River were received direct discharge of unusual high fresh water input with large amount of sediment plumes from mount Kinabalu during raining season [8] .
4.2. Spatial and Temporal Distribution of Nutrients Concentration
The range of nitrate concentration of 0.032 to 0.07 mg/l and range of phosphate concentration of 0.007 to 0.03 mg/l were recorded in Kota Belud’s coastal water. Comparable concentration of 0.019 to 0.09 mg/l of nitrate was determined coastal water of North-East Langkawi [12] . However, nitrate and phosphate concentration in Kuta Belud studied area were much lower compared to nitrate concentration (0.29 to 1.10 mg/l) and phosphate concentration (0.26 to 1.27 mg/l) in Sepanggar Bay [3] . Sepanggar Bay is well known with mariculture industry and contributed significant national fisheries production in Sabah [13] . In addition the anthropogenic activities around Seapnggar Bay much higher that contributes in nutrient concentration [3] than the Kota Belud’s area. Therefore, the nutrient enrichment in Sepanggar Bay is significantly higher than that in Kota Belud’s coastal water.
Nitrate concentration showed no significant different in transect A, C and D between dry and wet seasons. However, nitrate concentration in transect B (station B1 and B2) was relatively higher than that in nutrients concentration in other transects at wet season. Nutrients in these stations are influence by the surface runoff of surrounding areas and also down pour from the Tempasuk River. Tempasuk River is surrounded by mangrove forest, agriculture field, and Mount Kinabalu [8] . Therefore, Tempasuk River carried mangrove debris, agriculture wastes, and sediment plumes to coastal water. This observation concluded that higher nutrient concentrations occur in locations where freshwater entered after heavy rainfall events and washing nutrients from surrounding areas.
Phosphate concentration was similar in all station at dry season. It may be mentioned here that, the surface phosphate concentrations of the stations 0.5 km away from the coast line (A1, B1, C1 and D1) were higher during wet season compared to dry season. Nitrate and phosphorus loading is dependent on land use, fertilizer application, population density in addition to release from aquaculture activity [14] . Higher precipitation during wet season causes plenty of phosphate runoff from western part of Mt. Kinabalu as sediment plumes [8] . Thus, wet season can significantly increase the phosphate concentration in Kota Belud coastal water. However, stations located 10 km away from coast line showed no significant different in phosphate concentration. Thus heavy rain can introduce large quantity of nutrient but the distribution of nutrient will decrease with distance increases from land [15] .
4.3. Harmful Algal Bloom Community at Coastal Water of Kota Belud
Phytoplankton community of Kota Belud water was dominated by diatoms (93.51%) in term of genus density compared to Dinoflagellates (6.49%). Diatoms accounted for 77% of total phytoplankton in marine ecosystem [16] . A total number of nine HABs species were identified in Kota Belud’s coastal water where six species (Pyrodinium bahamense, Prorocentum micans, Neoceratium furca, Prorocentum sigmoides, Dinophysis caudate, and Neoceratium fursus) belongs to Dinophyceae and three species (Thalassionemanitzchoioides, Chatocerosaffinis, andRhizosoleniasp) belong to Bacillariophyceae. Chatoceros affinis (80.61%) was the dominant HABs species in Kota Belud’s water followed by Thalassionema nitzchoioides (12.76%) and Neoceratium furca (2.15%). Chatoceros affinis was found to be harmful in the Bay of Bengal [16] . Ceratium spp., Prorocentrum spp. and Peridinium spp. also are known to be responsible for red tides or other noxious algal blooms in Izmit Bay, Turkey [17] . In addition,Pyrodiniumbahamensehas been confirmed harmful in Sabah water [1] .
The cell density of Pyrodinium bahamense, the paralytic shellfish poisoning (PSP) producing Dinoflagellates, in the coastal water of Kota Belud was 3.36 × 103 cells/l at wet season. HABs consider occur when the cell density of dominant species is higher than 106cells/l for non-toxic production species and higher than 103 cells/l for toxic production species [1] . This indicates that the cell density of Pyrodinium bahamense at wet season was high enough to be considered as bloom. It is not surprise and Pyrodinium bahamense has been documented that grows well and bloom frequently in Sabah water throughout the whole year [6] . On the other hand, the cell densities of other HABs species were not high enough (<106 cells/l for non-toxic production species and <103 cells/l for toxic production species) to be considered as blooms.
4.4. Spatial and Temporal Distribution of HABs
Abundance of HABs was found to be highest (5.01 ± 0.50 × 104 cells/l) at station A1 which was located in front of Abai River that discharges fresh water from the western part of Mt. Kinabalu. Abai River not only carried sediment plumes from Mount Kinabalu but also nutrients from the large mangrove forest coverage along the Abai River [8] . The release of soluble inorganic nutrient (nitrate and phosphate) has a very high potential for HABs blooming.
Total HABs cell density in transect A (A1 and A2), C (C1 and C2) and D (D1 and D2) at wet season were significantly higher than that in HABs density recorded at dry season. Similar observation was documented in previous study where phytoplankton density was significantly higher during Northeast monsoon (26.38 × 106 cells/l) than Interseasonal monsoon (23.98 × 106 cells/l) in Sepangar Bay, Sabah [18] . This indicates that rainfall results in more nutrients runoff and might significantly increase of HABs density at coastal water.
Interestingly, although the nutrients concentration was significantly high in wet season than in dry season at transect B. But no significant difference was observed in HABs cell density at transect B (B1 and B2) between dry season and wet season. The reason of this was low salinity in transect B limiting the HABs bloom. In agreement with Anton et al. [19] and Usup et al. [1] , low salinity in Kimamis Bay, Sabah during heavy rainfall limiting the growth of Pyrodinium bahamense at Sepanggar Bay. Nutrient concentrations are not the only factor affecting the phytoplankton blooms because changing in environmental parameter might favor some species to blooms [20] . The environmental parameters such as temperature, salinity, Secchi depth (visibility), pH, and dissolved oxygen are the factors that can affect the algal cells community succession or limit the growth of certain groups of algae [20] . This suggests that salinity is an important factor that influence HABs in Kota Belud’s water other than nutrients concentration.
4.5. Influence of Nutrients Concentration on the Harmful Algal Blooms Cell Density
This study shows HABs densities were noticeably higher in higher nutrient levels. HABs have been known to occur often in nutrient-rich coastal water such as ports, estuaries, and bays [21] . High abundance of phytoplankton of 5.67 × 106 cells/l in cage culture area of Sepanggar Bay, Sabah was attributed to release of inorganic nutrients (nitrate and phosphate) [18] . This evident a relationship between excessive dissolve inorganic nitrogen (nitrate) and phosphorus (phosphate) loading and initiation of the HABS bloom.
This study shows Nitrate concentration showed stronger relationship with HABs density (R2 = 0.802) compared to the relationship between phosphate and HABs density (R2 = 0.514). Enrichment in nitrogen particularly nitrate lead to an increase in phytoplankton biomass in marine habitat [4] . This suggests that nitrate is the limiting factor in Kota Belud’s coastal water and HABs density is very sensitive to the changes of nitrate concentration. Therefore, nitrate is a more important factor than phosphate in Kota Belud’s water for HABs blooms.
5. Conclusions
This study has discovered that at least nine HABs or potential HABs species have been confirmed in Kota Belud water, namely Chatoceros affinis, Thalassionema nitzchoioides, Rhizosolenia sp., Pyrodinium bahamense, Prorocentum micans, Neoceratium furca, Prorocentum sigmoides, Dinophysis caudate, and Neoceratium fursus. Although 9 species were found in Kota Belud water year-round however only Pyrodinium bahamense bloom (cell density >103 cells/l for toxic production species) took place during this study.
Cell density of harmful algal bloom in Kota Belud was higher in wet season. Runoff after heavy precipitation initially resulted in increased accumulation of nutrients (nitrate and phosphate) concentration in the estuary. Increasing in nutrients concentration was generally associated with increasing of HABs cell density. This study also discovered that nitrate was limiting factor for HABs cells density rather than phosphate in Kota Belud’s coastal water.
Acknowledgements
This study was supported by University Malaysia Sabah. This work would not have been possible without the support of from Borneo Marine Research Institute staffs.
NOTES
![]()
*Corresponding author.