Recovery and Upgrading of Phosphorus from Digested Sewage Sludge as MAP by Physical Separation Techniques ()
Received 15 February 2016; accepted 9 May 2016; published 12 May 2016

1. Introduction
Phosphorus is one of the most important elements; it is very essential in agricultural sector as an efficient fertilizer. According to US Geological Survey, the world consumption of phosphorous in fertilizers in 2013 was around 40.7 million tons and it is expected to increase to 45 million tons in 2017 (US Geological Survey, 2014 [1] ).
Phosphate rocks are considered as the main source of phosphorus, and they are largely processed to produce phosphoric acid. There is no phosphate deposit in Japan, and all of the domestic consumption of phosphorus is covered by imported phosphate rocks. For this reason and because of the depletion of mineral phosphorus resources, it is imperative to find an alternative resource of phosphorus; in this regard, sewage sludge in which over than 90 % of waste water phosphorus is transferred into (Sebastian Petzet et al., 2012 [2] ), is considered as a secondary resource for phosphorus.
Although Sewage sludge can be directly used as fertilizer, its application to agricultural land is limited because of the content of toxic materials including organic compounds (E.Z. Harrison et al., 2006 [3] ), pathogenic microorganisms (G. Carbonell et al., 2009 [4] ) and heavy metals (F. Laturnus et al., 2007 [5] ).
Anaerobic digestion is extensively used to treat sewage sludge (Hamzawi, et al., 1998 [6] ), in Japan as an example; there are 300 sewage treatment plants, i.e. one-fourth of the 1200 sewage treatment plants implemented in Japan use anaerobic digestion to treat sludge. Anaerobic digestion of mixed raw sludge produced during sewage treatment reduces the sludge amount and enables the recovery of methane gas. Solids included in the sludge are digestion residues, methanogens, other microorganisms, and inorganic matters etc., while dissolved matters are ,
, 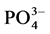 ,
, 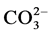 among others. Digested sludge is dewatered and the solid portion is turned into sludge cakes. Careful observation of such cakes reveals the existence of shiny crystals of about 0.1 - 2.0 mm in size, scattered throughout the surface. These are magnesium ammonium phosphate (MgNH4PO4∙ 6H2O) particulates, commonly known as MAP or struvite. MAP as a slow release fertilizer, had been evaluated in terms of fertilizer quality and showed high quality results (Ghosh et al., 1996 [7] ).
among others. Digested sludge is dewatered and the solid portion is turned into sludge cakes. Careful observation of such cakes reveals the existence of shiny crystals of about 0.1 - 2.0 mm in size, scattered throughout the surface. These are magnesium ammonium phosphate (MgNH4PO4∙ 6H2O) particulates, commonly known as MAP or struvite. MAP as a slow release fertilizer, had been evaluated in terms of fertilizer quality and showed high quality results (Ghosh et al., 1996 [7] ).
Several technologies have been developed to recover phosphorus whether from liquid phase or from sewage sludge. Recovery of phosphorus from liquid phase is mainly based on precipitation; Toyama, et al., 2012 [8] developed a new method to precipitate phosphorus from sewage sludge by carbon dioxide blowing, precipitation as hydroxyapatite by the addition of calcite (Song et al., 2006 [9] ) or by the addition of calcium hydroxide (Hosni et al., 2008 [10] ). Phosphorous can be recovered by adsorption on zirconium adsorbate and then the desorbed phosphorus was precipitated as trisodium phosphate (Ebie et al., 2008 [11] ), precipitation of phosphorus as struvite or MAP is applied on a broad scale.
Recovering of phosphorus from solid sludge is achieving through thermochemical processing (incineration) of sewage sludge; this process reduces the volume by 80% - 90% and is followed by leaching and recrystallization to recover phosphorus from the incinerator ash, leaching can be done by acids (Biswas, et al., 2009 [12] ) or alkalis (Sano, et al., 2012 [13] ) or by combining acids and alkalis (Petzet, et al., 2012 [2] ). Leaching is widely implemented to recovery phosphorus despite its high chemical consumption (Franz, et al., 2008 [14] ).
Most of the above mentioned technologies are not economically sound in terms of chemicals, energy and byproduct treatments costs. That might be why phosphorus recovery from sewage sludge has not become widely spread.
Hagino et al., (2005 [15] ) have succeeded to recover about 90% of phosphorus as MAP (Magnesium ammonium phosphate) from anaerobic digested sludge by physical separation techniques using apparatuses such as a vibrating screen, a 4-inch hydrocyclone, and a Multi-Gravity Separator (MGS). MAP exists naturally in anaerobic digested sludge, so it is not necessary to add agents or control pH of water. So, with the above-mentioned apparatuses, it is supposed that phosphorus can be recovered at an economic price. But the collected MAP products contain traces of heavy metals with a specific gravity similar or higher to that of the MAP. In this study, our primary target is to establish the system for recycling of phosphorus, with a particular emphasis on removal of heavy metals like Cd, Pb, Ni, Cr and As from MAP products using magnetic separator to comply with Japan standards for fertilizers (Forestry and Fisheries of Japan, 2006 [16] ).
2. Materials and Method
2.1. Sewage Sludge Samples
Tests were carried out on anaerobic digested sludge supplied from two sewage-treatment plants (plant A and plant B). The properties of the digested sludge of the two plants are shown below in Table 1.
2.2. Recovery and Upgrading of MAP
The illustrated scheme in Figure 1 was used to recover and upgrade MAP from the digested sludge. As seen, MAP in sludge was concentrated through physical separation apparatuses such as a vibrating screen, a 4-inch hydrocyclone and a Multi-Gravity Separator (MGS). In plant A, a 2-inch hydrocyclone was used after concentration by a 4-inch hydrocyclone, the characteristics of these apparatuses are given below in Table 2.
Magnetic separators were used to remove heavy metals from the recovered MAP; the MAP recovered from plant A (MAP-A) was tested through a dry magnetic separator (Type G-30 + 30, NIPPON MAGNETIC DRESSING CO., LTD) while the one recovered from plant B (MAP-B) was submitted to both dry and wet magnetic separators (Type NJ, NIPPON MAGNETIC DRESSING CO., LTD). The tested magnetic flux density varied from 5000 to 20,000 Gauss. The upgraded MAP is collected as nonmagnetic product.
The grade of MAP in the MGS products, as well as in the nonmagnetic fraction, was assessed through the concentration of phosphate ion (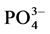 ) in the sulfuric acid solution derived from these products and analyzed by
) in the sulfuric acid solution derived from these products and analyzed by
![]()
Table 1. Properties of digested sludge.
TS: total solid, SS: suspended solid.
![]()
Figure 1. Scheme of separation and upgrading of MAP.
a spectrophotometer (UV-2450, Shimadzu). Minerals contained in MAP products (MGS products), as well as nonmagnetic and magnetic products, were detected by XRD (RINT2100, Rigaku) and polarizing microscope observation. Heavy metal contents of MAP products and nonmagnetic products were also determined by ICP-AES (VISTA-MPX, Seiko instrument Inc.). Spectrophotometer and ICP-AES measurements were carried out three times for more accuracy. SEM-EDX (SS-550, Shimadzu) analysis was carried out on magnetic products of MAP-A.
To evaluate separation efficiency of magnetic separation experiments, Newton’s efficiency was calculated according to the following equation:
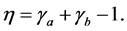
where η is Newton’s efficiency; γa is the recovery of MAP in the nonmagnetic fraction; and γb is the recovery of non-MAP in the magnetic fraction.
3. Results and Discussion
3.1. Recovery of MAP
The results of MAP recovery for plants A and B at every stage are shown in Table 3, Table 4 respectively.
At vibrating screen, coarse solid impurities with a particle size exceeding 1.0 mm were removed and the MAP recovery was 99.3%, 98.9% for plant A and B respectively. Hydrocyclones were used to increase MAP’s pulp density to about 20% - 30% as a prerequisite for multi gravity separator (MGS), the recoveries at this stage were 93.3%, 95.9% for A and B respectively. Till this stage, the change in MAP grade was very slight. But the dramatic change in the grade achieved with the aid of MGS apparatus; MAP-A grade increased from 0.08% to 88.9% with 90.4% recovery and the grade of MAP-B increased from 0.11% to 73.8 with 93.2% recovery. The increase in the grade is attributed to removal of organic matters by the washing effect of MGS.
3.2. Upgrading of MAP by Magnetic Separation
The collected MAP products contain impurities such as organic materials and, particularly, heavy metals that must be removed to comply with Japan standards for fertilizers.
In the following section, dry and wet magnetic separation tests were carried out at different magnetic flux densities to remove these impurities from the MAP products.
![]()
Table 3. Batch and overall recovery of MAP-A.
![]()
Table 4. Batch and overall recovery of MAP-B.
3.2.1. Appropriate Magnetic Force
As illustrated in Figure 2 it was found that the yield of magnetic products increased with the magnetic flux density. The impurities in MAP products were removed efficiently at high magnetic flux density. Besides, wet separation was found to be less effective than dry separation.
The grade of MAP in products is reported in Table 5. On dry magnetic separation tests, the grade of MAP in the nonmagnetic products is the highest at 15,000 Gauss, while the yield of magnetic products at 20,000 Gauss is higher than the one obtained at 15,000 Gauss. According to these results, it is estimated that MAP loss increased over 15,000 Gauss. About wet separation, it is not necessary to raise the magnetic force to 28,000 Gauss since the grade of MAP in non-magnetic products goes down.
The Newton’s efficiency of each process was calculated to determine the optimal magnetic flux density. It was found that dry separation is most efficient at 15,000 Gauss, while the most appropriate magnetic force for the wet magnetic separation is 20,000 Gauss.
3.2.2. Mineral Composition of Collected Products
The XRD spectra of MGS products and nonmagnetic products at 15,000 Gauss are shown in Figure 3. As illustrated, peaks of struvite (MAP) appear chiefly in all the investigated samples. In comparison to MAP-A before magnetic separation, the intensity of struvite peaks in nonmagnetic products is found to have increased. As
![]()
Figure 2. Yield of magnetic products (d): dry magnetic separation, (w): wet magnetic separation.
for MAP-B, there is not much difference between dry separation and wet separation.
According to the results of XRD analysis, it was found that the magnetic product of MAP-A contains magnetite, quartz, pyroxene, plagioclase, and so on. It means that not only magnetic materials but also nonmagnetic materials have been removed from MGS product by magnetic separation. This fact has been confirmed by polarizing microscope examination through which the presence of amphibole was also detected. Magnetic products of MAP-B, separated through both dry and wet magnetic separators, were estimated to contain manganocummingtonite. In the wet separation process, it is noticed that muscovite is collected in the magnetic product.
The table (Table 6) summarizes XRD results for the feed and magnetic separation products.
![]()
Figure 3. XRD spectrum of collected MAP products. (a) XRD spectrum of MAP-A; (b) XRD spectrum of MAP-B.
![]()
Table 6. Phases distribution for magnetic separation fractions according to XRD results.
Ø: contains too much, ○: contains, ∆: contains too little, and ×: can’t be detected.
3.2.3. Removal of Heavy Metals
The contents of heavy metals contained in samples are shown in Figure 4. Values reported on the bars are the official specifications of fertilizer defined by the Ministry of Agriculture, Forestry and Fisheries of Japan (2006). It was found that MAP-A meets the illustrated standard even without passing through a magnetic separator. With magnetic separation, the contents of heavy metals in both MAP-A and MAP-B decreased, it means that magnetic separation is effective for the removal of heavy metals. The content of Pb in MAP-B was over the standardized range, but it decreased after magnetic separation. In comparison with wet magnetic separation, dry magnetic separation is more effective for the removal of Cr and Ni. But more Pb could be removed by wet separation rather than dry separation process.
According to ICP-AES analysis, the content of Fe and Ti in MAP decreased. Besides, some other metals such as Al, Ba and Ca, which are supposed to be components of nonmagnetic materials, are also removed by the magnetic separators. This is in agreement with the results of XRD analysis. The reason why nonmagnetic materials can be removed through magnetic separation is unknown. It is expected that fine particles of magnetic materials like magnetite might adhere to the surface of nonmagnetic materials such as plagioclase, and make them behave like magnetic materials.
To confirm the above assumption, SEM-EDX analysis was carried out. Figure 5 shows one of the nonmagnetic material particles found in magnetic products of MAP-A. It contains chiefly Si, O, Mg, and is supposed to be plagioclase. It was observed that many particles have adhered to the surface of the sample. The particles were too fine to detect their nature, but, as Fe is collected into magnetic products, according to the ICP-AES results, these particles are estimated to be iron mineral such as magnetite or pyrrhotite. In order to remove heavy metals more effectively, it is necessary to carry out further investigations on the mechanism that makes nonmagnetic materials to be recovered as magnetic materials.
4. Conclusions
In order to develop a system for the recovery of phosphorus from anaerobic digested sludge, separation appara-
![]()
Figure 4. Contents of heavy metals in MAP products. (a) MAP-A; (b) MAP-B.
![]()
Figure 5. A nonmagnetic material in magnetic product. (a) ×450; (b) ×2000.
tuses such as a vibrating screen, a 4-inch hydrocyclone and a MGS were tested in sewage-treatment plants A and B. As MAP products obtained after MGS treatment contain heavy metals, dry and wet magnetic separation processes were tested for their removal. It was found that the yield of magnetic products, as well as the loss of MAP, increased with the magnetic force. However, according to the grade analysis, magnetic separation was found to be effective for the improvement of the grade of MAP, and the most appropriate magnetic flux density was 15,000 Gauss for a dry separator, and 20,000 Gauss for a wet separator.
XRD spectrum shows that MGS products and nonmagnetic products consist chiefly of struvite. The results of the ICP-AES analysis indicate that magnetic products contain not only magnetic materials but also nonmagnetic materials with induced magnetic properties. This induced magnetism might result from the adhesion of fine magnetic particles to the surface of nonmagnetic materials. Both dry and wet magnetic separation processes were found to be able to remove heavy metals from MAP.
Acknowledgements
This work was partially supported by a Grant-in-Aid for Science Research (JSPS KAKENHI Grant No. 15H02333) from the Japan Society for the Promotion of Science (JSPS), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
NOTES
![]()
*Corresponding author.