Nitrogen Application Rate and Genotype Effects on Switchgrass Production and Chemical Characteristics ()
Received 18 December 2015; accepted 20 March 2016; published 23 March 2016

1. Introduction
Biofuel crops are expected to contribute substantially towards energy security, environmental stewardship, and economic gains in the USA. Environmental benefits to be realized include greenhouse gas reduction, carbon- neutral biofuel production, use of and reclamation of marginal lands and relatively low-input production systems [1] . Switchgrass was selected for research and development as a dedicated bioenergy crop for the lignocellulosic biofuel industry in 1992 by the U.S. Department of Energy’s Bioenergy Feedstock Development Program [2] . Optimizing and sustaining bioenergy crop production systems requires knowledge of the system itself and factors that have an effect on sustainable productivity.
Nutrient uptake and loss from production sites are important issues for high biomass producing crops such as switchgrass. Nitrogen has an effect on crop growth, development, and physiological processes and determines crop productivity, chemical characteristics, and profitability. It is one of the most challenging nutrients to manage effectively. In many agroecosystems, a very substantial portion of applied N is lost from soil to ground water, rivers, and oceans [3] [4] , because crop plants only convert 30% - 40% of this applied N to useful products [5] . In lignocellulosic crops, it is important to minimize feedstock nutrient concentration that may contribute to ash at combustion causing slagging and fouling of processing equipment as well as reduced efficiency of ethanol conversion processes. Therefore, a high quality biofuel feedstock should have minimal moisture, low N and ash contents, minimal undesirable mineral concentration with high cellulose [6] [7] . This requirement will require judicial management of N application during crop production.
Nitrogen removal in the biomass can determine the fertilizer requirements of switchgrass [8] . The quantity of N removed in harvested biomass depends on the genotype, quantity of N applied, harvest frequency and timing, and temporal weather variability. Lowland cultivars are more responsive to applied N [9] [10] , later maturing, and produce greater biomass than upland cultivars in many southern US environments.
Removal of N and other nutrients is a function of biomass yield and tissue N concentration [11] . Incremental increases in N application invariably increase switchgrass biomass yield and tissue N concentration [12] . The magnitude of increase usually follows the law of diminishing returns with the response to incremental increase being progressively less with each increase in N application [13] . At 120 kg N ha−1, the amount of N removed was similar to the amount of N applied and rates above this level led to increased soil nitrate-N levels [11] . In Alamo, N removal was 50% greater than the amount of N applied, attributed to the ability of switchgrass to sequester N possibly through symbiotic relationships with soil or plant microbes [9] . Accumulation of P, K, and other plant nutrients in switchgrass is additional factors in understanding sustainable production of quality feedstock. These parameters and their interactions with N application and switchgrass genotypes have not been studied extensively.
Management decisions effects on yield and nutrient balance of production systems as well as on biomass quality and ethanol yield also have not been adequately addressed. Biomass feedstock composition varies with harvest date, location, and fertility management [13] . Previous reports on biomass quality reported mainly cell wall composition traits but not characterization of ethanol components or quantification of ethanol yield [6] [7] . Ethanol production via fermentation can be achieved from direct conversion of sugars and enzyme-mediated hydrolysis of starch or cellulose. Lignocellulose is a complex of cellulose, hemicellulose and lignin and therefore requires additional process steps for ethanol conversion. These steps include delignification to release cellulose and hemicellulose, depolymerisation of cellulose and hemicellulose to produce fermentable sugars before fermentation to produce ethanol. Simultaneous saccharification and fermentation (SSF) is one process option for the production of ethanol from lignocellulosic biomass and integrates the enzymatic hydrolysis of cellulose to glucose and fermentation of glucose to ethanol. The SSF procedure requires complex fermentation assays and compositional analysis of biomass. With the development of near-infrared spectroscopy (NIRS), however, it has become analytically acceptable to determine biomass composition using equations developed by the USDA- ARS NIRS Consortium [14] . This experiment quantified the effects of N rates and switchgrass genotypes on nutrient removal rates, chemical composition, and ethanol yield. These findings will have direct implications for nutrient management and feedstock quality control in switchgrass production systems in the U.S. Midsouth.
A systematic approach to nutrient management requires an understanding of nutrient removal capacities, biomass production and feedstock quality. Understanding and quantifying nutrient removal in switchgrass production systems can aid in cultivar selection, feedstock quality control, optimized agronomic resource management, and reduction in nutrient loss. Quantitative information required for optimizing nutrient dynamics, yield, and quality in switchgrass is incomplete and inadequate. The objective of this study was to determine the effects of N application and genotypes on biomass production and nutrient removal, including micro-nutrients, and biomass and ethanol yield in switchgrass grown in a site that represents the U.S Midsouth.
2. Materials and Methods
2.1. Study Site
This field study was planted in May 2006, and treatment imposition and data collection were conducted from 2008 through 2009 growing seasons at the Brown Loam Branch Experiment Station, Raymond, MS (32˚15'N, 90˚30'W). The soil at the site is a Loring silt loam (fine-silty, mixed, thermic Typic Fragiudalfs) characterized by 2% to 5% slopes, eroded, moderately well-drained with a fragipan.
2.2. Weather Conditions
Weather data were collected from the climate station located at the research site. Growing season (April-No- vember) precipitation for 2008 (1023 mm) and 2009 (955 mm) was more than the 30-yr average (924 mm). March 2008 precipitation (71 mm) was the driest March recorded at the location, while March 2009 (235 mm) precipitation was 230% above March 2008 and 69% more than the 30-yr average March precipitation (139 mm) (Table 1). Mean air temperature for 2008 (21.6˚C) and 2009 (21.9˚C) was comparable to the 30-yraverage temperature of 21.8˚C (Table 1).
2.3. Treatments and Experimental Design
Following seedbed preparation in May 2006, genotypes were seeded with a small-plot planter (Kincaid Equipment and Manufacturing, Haven, KS) at a rate of 5.6 kg pure live seed ha−1 in seven rows 22 cm apart. The experiment was a split-plot arrangement of a randomized complete block design with four replications. Treatments were switchgrass genotypes (three related lowland genotypes: Alamo, NF/GA001, NF/GA992 and one upland genotype: Cave-in-Rock) as whole plots and N rates (0, 80, 160, and 240 kg∙ha−1 N) as subplots. In late fall of each year, (including the year prior to the initiation of this experiment) all plots were harvested at a10-cm stubble
![]()
Table 1. Monthly precipitation and mean monthly air temperature for 2008 and 2009 and the 30-yr average (1979-2009) at the Brown Loam Branch Experiment Station, Raymond, MS.
height. In early May of both study years, fertilizer was applied to subplots according to treatment in a single broadcast application of urea (46-0-0) by hand. No chemical weed and pest control or supplemental irrigation was applied during the experiment period.
2.4. Data Collection
To determine biomass yield, a 1 × 0.5 m area in the center of each subplot was cut to a 10-cm stubble height in November of each year using 7.2 volt cordless battery-operated hand-held shears (Stanley Black and Decker, New Britain, CT). The total harvested material was weighed fresh and an approximately 1-kg subsample was taken and dried in a forced-air oven at 55˚C to 60˚C for 72 h or until constant weight was achieved in order to determine dry matter (DM) concentration. A second subsample taken from the harvested material was dried similarly and ground to pass a 1-mm stainless steel screen using a Wiley Mill (Model 4; Thomas Scientific, Swedesboro, NJ). Analysis of N, P, K, Ca, and Mg was done at the Mississippi State University Soil Testing Laboratory. A homogenized subsample was analyzed for fiber constituents and sugar composition by NIRS using a FOSS NIRSystems Model 6500 spectrophotometer (FOSS Industries, Silver Spring, MD). Samples were scanned using the FOSS ISIscan software Version 4.4 (Infrasoft International) with grass hay and switchgrass prediction equations (Vogel et al., 2011) developed by the NIRS Forage and Feed Testing Consortium (Hillsboro, WI). Calibration statistics used were developed by Vogel et al. [14] . Hemicellulose and cellulose concentrations were calculated as the difference between predicted neutral detergent fiber (NDF) and acid detergent fiber (ADF) concentrations and the difference between predicted ADF and acid detergent lignin (ADL) concentrations, respectively.
2.5. Calculations and Statistical Analysis
Nutrient removal was calculated by multiplying the biomass yield by elemental tissue concentration. Nitrogen use efficiency (NUE; kg biomass kg−1 N) was calculated as the ratio of the increase of biomass harvested that resulted from N fertilizer application to the 0 N control. Apparent N Recovery (ANR; %) was calculated as the ratio of N removed from N fertilizer application to the 0 N control expressed as a percent.
Ethanol yield was calculated following the methods of Vogel et al. (2011) who determined ethanol per gram dry forage (ETOH; mg∙g−1) and pentose sugars released per gram dry forage (mg∙g−1) from SSF procedures. Actual ethanol yield via SSF was calculated using the ETOH result. In this procedure, the yeast used to ferment the released sugars cannot ferment pentose sugars, so ETOH was only from released hexoses. Given this, the equation below to calculate total ethanol yield (TEY) accounts for ethanol yield from SSF-released glucose from biomass using the calculation ETOH × 1.267, and the theoretical ethanol yield (TTEY) from pentose sugars, arabinose (ARA), and xylose (XYL) using the calculation (ARA + XYL) × 0.579 × 1.267.
Total ethanol yield (L∙Mg−1) is a summation:

The TTEY from all biomass hexoses considers concentrations of ARA, fructose (FRU), galactose (GAL), glucose (GLC), mannose (MAN), soluble glucose (GLCS), starch (STA), and sucrose (SUC) and was calculated (assuming 100% conversion) as [(MAN + GAL + GLC + STA) × 0.57 + (GLCS + FRU) × 0.51 + (0.537 SUC) × 1.267]. Theoretical ethanol yield from pentose sugars was calculated as (ARA + XYL) × 0.579 × 1.267.
The equation to calculate TTEY (L∙Mg−1) is the summation of the two:
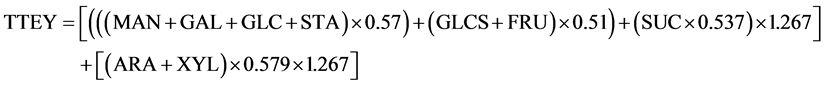
Total ethanol production (TEP) was calculated as TEY × biomass yield. Similarly, total theoretical ethanol production (TTEP) was calculated as TTEY × biomass yield.
The data were analyzed by fitting mixed models with repeated measures using PROC MIXED of SAS [15] . Genotype, N application rate, year, and their interactions were considered fixed effects while replication × year was considered a random effect. Year was considered as a repeated measure and responses were considered different at the 0.05 probability level. Means were separated using the PDIFF option. Relationships among biomass yield, fiber properties, and sugar composition were explored using correlation analysis. When correlations were detected, regressions were performed using PROC REG of SAS to quantify the relationship.
3. Results and Discussion
3.1. Biomass Yield
There was a year × N rate interaction (P = 0.009) effect on annual biomass yield. Generally, biomass yield during 2009 was greater than 2008; however, the responses within year were different (Figure 1). In 2008, biomass yield increased linearly with N rate (P = 0.013), but in 2009, the response was quadratic (P < 0.001). There was also a year × genotype interaction effect on annual biomass yield (P = 0.019). The interaction occurred partly because of differences in the patterns of means separation among genotypes within years. In 2008, NF/GA001 (9.3 Mg∙ha−1), NF/GA992 (8.4 Mg∙ha−1) and Alamo (7.9 Mg∙ha−1) had similar biomass yield but in 2009, NF/ GA001 (13.8 Mg∙ha−1) and NF/GA992 (15.0 Mg∙ha−1) were greater than that of Alamo (12.6 Mg∙ha−1). Cave-in- Rock biomass yield was always less (4.6 and 6.3 Mg∙ha−1 in 2008 and 2009, respectively) than the other genotypes. Other studies have reported variation in biomass yield responses across years that vary markedly [8] [12] [16] .
Biomass yield increases ranged from 58% [8] to 85% [7] in the second year of harvest in 1-yr switchgrass stands. Data from those studies indicate that response trends for biomass yield with age of stand are similar across geographic locations although absolute values vary. Differences in absolute values of responses observed across location may be attributable to early-season precipitation variability between years [17] , inherent soil physical and chemical properties, photoperiodism, harvest frequency, as well as age-related stand productivity potential. Interannual biomass variability is correlated with growing season rainfall [12] . Switchgrass biomass was reported to increase with stand age up to 3 yr, plateau at around 4 to 5 yr and decline thereafter [8] [12] [18] . The biomass yield differences between years observed in this study may have been influenced, in part, by the greater precipitation in March 2009.
Averaged across genotypes, biomass yield increased by 29% (2008) and 69% (2009) at 80 kg N ha-1 compared to the control, but there was no further increases with the additional N rates (Figure 1). Lowland cultivars are more responsive to N application and have greater growth potential than upland cultivars due to mid-season growth vigor and delayed maturity [10] . In southern locations such as in this study, Cave-in-Rock, a northern
![]()
Figure 1. Year × N rate interaction effects on biomass yield of switchgrass grown during 2008 and 2009 at the Brown Loam Branch Experiment Station, Raymond, MS.
upland variety, is expected to produce less than Alamo and the experimental lines (NF/GA001 and NF/GA992) [10] [19] . The breeding lines NF/GA001 and NF/GA992 are selections from parental Alamo populations, possibly explaining why there was no difference in biomass yield response to N application rate among these three related genotypes.
3.2. Tissue Nutrient Concentrations
There was no effect of genotype, N rate or their interactions on tissue N concentration (P > 0.5), which ranged from 7.16 to 8.15 g∙kg−1 with a mean of 7.72 g∙kg−1 (data not shown). Other studies have reported that tissue N concentration increased with N application rate (Guretzky et al., 2011; Jung et al., 1990; Madakadze et al., 1999). The average tissue N concentration (7.64 g∙kg−1) among genotypes at the zero N rate was within range of 8.2 g∙kg−1 reported by Jung et al. [20] but more than 4.3 g∙kg−1 reported by Guretzky et al. [17] when no N was applied. These differences may result from inherent soil physicochemical properties, the plants ability to sequester N deeply in the soil profile through its extensive root system, and time of harvest. Since N is mobile in the plant and is translocated from shoots to roots at the onset of senescence, harvesting after senescence will reduce tissue N concentration [16] .
There was no effect of N rate on P, K, Ca, Mg, and S tissue concentrations (P > 0.5); however, there was a year × genotype interaction on P, K, Ca, and Mg concentrations (P < 0.05). Tissue P concentration of Cave-in-Rock and NF/GA992 decreased from 2008 to 2009 (Table 2) but did not change with year in the other two genotypes. Across years and genotypes, P concentration ranged from 0.33 to 0.85 g∙kg−1, compared to the 0.7 to 0.9 g∙kg−1 reported by Guretzky et al. [17] and 0.90 to 1.50 g∙kg−1 reported by Propheter et al. [21] . Tissue K concentration was greater in 2008 than 2009 (Table 2). In 2008, K concentration was greater in Cave-in-Rock than the other genotypes but was similar across genotypes in 2009. Tissue P and K concentrations were less than those reported previously [17] [21] . Tissue Ca concentration within all genotypes was greater in 2008 than 2009 and among genotypes within both years, was greater for Cave-in-Rock than the other genotypes (Table 2). Tissue Mg concentration in 2008 was greater than 2009 for all genotypes except NF/GA992, which had similar concentration both years. Among genotypes, Mg concentrations were similar in 2008 but the magnitude of decrease in 2009 was larger for Cave-in-Rock than the other genotypes, resulting in it being the least among genotypes (Table 2). Tissue S concentration was greater in 2008 than 2009 (Table 2). We inferred from the results that N fertility did not have an effect on tissue macronutrient concentrations in switchgrass grown in a fertile soil. Production using a particular genotype probably will require specific nutrient management in soils with low levels of plant available nutrients.
3.3. Nutrient Removal
There were N rate (P = 0.0009), genotype (P < 0.0001), and year (P = 0.0184) main effects on N removal but no
![]()
Table 2. Means of P, K, Ca, Mg, and S tissue concentrations among switchgrass genotypes during the 2008 and 2009 growing seasons at Brown Loam Branch Experiment Station, Raymond, MS.
†Within column, means followed by the same lowercase letters, and within rows, means followed by the same uppercase letters are not different (P > 0.05) using the pairwise difference option (PDIFF) in SAS.
interactions (P > 0.05) were detected. Among genotypes, N removal followed the same pattern as biomass yield responses. Among Alamo, NF/GA001 and NF/GA992, N removal was quadratic in response to N application rate (y = 74.44 + 0.59x ? 0.002x2, r2 = 0.80) and linear for Cave-in-Rock (y = 32.51 + 0.123x; r2 = 0.99) (Figure 2). Averaged across genotypes and N rate, 2009 N removal (99.4 kg N ha−1) was greater than 2008 (72.5 kg∙ha−1). The increase in N removal across years appears to be related to the 54% increase in biomass yield from 2008 to 2009.
There was no effect of tissue N concentration on N removal, possibly because of post maturity shoot to root translocation of N [16] . Jung et al. [20] reported that N removal increased by 69% with application of N from 0 (48.8 kg∙ha−1) to 75 kg∙ha−1 (81.88 kg∙ha−1) and this difference was related to biomass yield and tissue N concentration. For Alamo, NF/GA001, and NF/GA992, N removal was 112% greater than that of Cave-in-Rock (Figure 2).
There was a year × genotype interaction (P = 0.0284) on P removal (Table 3). Between years, P removal was
![]()
Figure 2. Genotype × N rate interaction effects on biomass yield of four switchgrass genotypes grown during 2008 and 2009 at the Brown Loam Branch Experiment Station, Raymond, MS.
![]()
Table 3. Means of P, K, Ca, Mg, and S removal rates among switchgrass genotypes and N rates during the 2008 and 2009 growing seasons at Brown Loam Branch Experiment Station, Raymond, MS.
†Within column, means followed by the same lowercase letters, and within rows, means followed by the same uppercase letters are not different using PDIFF in SAS (P > 0.05); ‡OPC, orthogonal polynomial contrast (L, linear; Q, quadratic); *, **, ***, significant at the 0.05, 0.01, and 0.001 levels, respectively; NS, not significant.
similar for all genotypes except for NF/GA001, which had 68% greater P removal in 2009 than 2008. In 2008, NF/GA992 had the greatest P removal, while in 2009, Alamo, NF/GA001, and NF/GA992 had greater P removal than Cave-in-Rock (Table 3). There was no N rate effect on P removal; contrary to previous findings where P removal rates tended to increase with N rate [17] . There was a positive correlation between P removal and tissue P concentration (P < 0.0001).
There were genotype (P = 0.0280) and year (P = 0.0014) main effects on K removal but no N rate effect (P > 0.05; Table 3), similar to previous findings [17] . In 2009, K removal was 76% less than 2008 for all genotypes (Table 3). Cave-in-Rock K removal was less than the other genotypes in both years. These removal rates are less than those reported by Guretzky et al. [17] .
Potassium removal was correlated with tissue K concentration (P < 0.0001). Calcium and Mg removal responded to N rate main effects (Table 3). Calcium removal increased by 51% from 0 to 80 kg applied N ha−1, but further N applications did not result in increased Ca removal (Table 3).
Similarly, Mg removal increased by 60% as N application increased from 0 to 80 kg N ha−1. Quadratic responses to N were observed for Ca (P = 0.0004, r2 = 0.99) and Mg (P = 0.0484, r2 = 0.95) concentrations.
Calcium removal was not correlated with Ca tissue concentration, however, Mg removal and Mg concentration were correlated (P < 0.0001). There was a genotype main effect on S removal (P = 0.0092). Cave-in-Rock S removal was 55% less than the average of the other genotypes. A positive correlation was found between S removal rate and tissue S concentration (P < 0.0001).
Nutrient removal rates in the biomass can be used to estimate fertilizer requirements of switchgrass [8] . Management practices such as cultivar selection [9] [10] , N fertilizer application rate [12] [22] , harvest frequency [8] [23] , and harvest timing [8] [9] [22] may have an effect on the magnitude of nutrient removal from production systems and can be minimized by adopting the optimum combination of management factors.
3.4. Nitrogen Use Indices
There was genotype × N rate interaction on both NUE (P = 0.0003) and ANR (P = 0.0265). Nitrogen use efficiency has been used to describe a plant’s capacity to acquire and utilize N for producing biomass and is expressed as the biomass yield produced per unit N applied. NUE decreased monotonically above 80 kg∙ha−1 N for all genotypes, except for Cave-in-Rock. Among genotypes, NUE varied in response to N application rate (P< 0.0001) with a linear response observed for Cave-in-Rock and quadratic responses for Alamo, NF/GA001 and NF/GA992 (Figure 3(a)). Averaged across genotypes, NUE declined by 39% from 80 to 160 kg N ha−1 and further decreased by 23% from 160 to 240 kg N ha−1. At 80 kg N ha−1, NUE for Cave-in-Rock was 50% less than other three genotypes (Figure 3(a)).
Alamo and the two experimental lines derived from Alamo, NF/GA001 and NF/GA992, therefore, were more efficient in utilizing N and converting to biomass production at this location. There were no further benefits to biomass yield at N rates above 80 kg ha−1 N, as a result, NUE decreased with increasing N application rates. Apparent N recovery responses were similar to NUE, that is, decreased with increasing N rate (Figure 3(b)). Among genotypes, ANR varied in response to N application rate (P < 0.0001) with a linear response observed for Cave-in-Rock and a quadratic response for Alamo, NF/GA001, and NF/GA992. Lemus et al. [7] reported 80% N recovery at 90 kg ha−1 N during a 3-yr period. This was more than N recovery rates found in our study for Alamo and the experimental lines at 80 kg∙ha−1 N, possibly in part due to the lesser biomass production and N removal. Stout et al. [24] reported lower N recovery rates of 31% and 23% for 90 and 180 kg∙ha−1 N, respectively. In our study, at 80 kg∙ha−1 N Cave-in-Rock recovered 41% less N than the average recovery of the other three genotypes. Applications of N above 80 kg∙ha−1 reduced recovery rates and may increase the potential for N leaching. Relatively less biomass yield and N recovery suggests that Cave-in-Rock is not suitable as a feedstock in the U.S. Midsouth.
3.5. Cell Wall Constituents
Acid detergent fiber, lignin, and hemicellulose concentrations were different among genotypes (P < 0.05) but there was no effect of N application rates (P > 0.05). There was a year × genotype effect on NDF (P = 0.0146) and cellulose (P = 0.0266) concentrations. Among genotypes, ADF was greatest in Cave-in-Rock, followed by the experimental lines and Alamo (Table 4). Hemicellulose and ash concentrations were the least in Cave-in-Rock and the greatest in NF/GA992 but similar in Alamo and NF/GA001. This interaction indicates that
![]()
Figure 3. The effect of N application rate on (a) N use efficiency (NUE) and (b) apparent N recovery (ANR) of switchgrass averaged across 2008 and 2009 growing season, at the Brown Loam Branch Experiment Station, Raymond, MS. The symbols indicate the NUE and ANR across genotypes and the lines are fitted lines using a quadratic function for lowland genotypes and a linear function for upland Cave-in-Rock for NUE. The lines for ANR are fitted using a quadratic function for lowland genotypes and a linear function for upland Cave-in-Rock. Data are means and ± SE of four replications.
![]()
Table 4. Cell wall components and ash of switchgrass genotypes during the 2008 and 2009 growing seasons at Brown Loam Branch Experiment Station, Raymond, MS.
†ADF = acid detergent fiber; NDF = neutral detergent fiber; ‡Within column, means followed by the same lowercase letters, and within rows, means followed by the same uppercase letters are not different (P > 0.05) using PDIFF in SAS.
cell wall properties may change in different years in response to precipitation and temperature with genotypes responding dissimilarly.
Lignin concentration was greatest in the earliest maturing Cave-in-Rock (Table 4). These lignin results are greater than those reported previously [13] . Across all genotypes, NDF and cellulose increased by 6% and 10% from 2008 to 2009 (Table 4). Harvesting switchgrass after frost kill produced relatively large ADF and NDF concentrations, both highly desirable biofuel traits. Lowland genotypes had the greatest biomass yield and generally had the least lignin concentration, therefore may be more suitable for feedstock production in the U.S. Midsouth.
Ash concentration was different among genotypes (P = 0.0002). Cave-in-Rock had the least ash concentration while lowland NF/GA992 had the greatest ash concentration, suggesting that greater biomass yield led to an increase in ash concentration (Table 4). These results are contrary to previous reports [13] where lowland genotypes had the least ash concentration relative to upland genotypes. Nitrogen application rate did not have an effect on the cell wall composition in our study, supporting findings of Guretzky et al. [17] of only 1% and 2% increase in ADF and NDF as N rate increased from 0 to 225 kg N ha−1 after a frost harvest for Alamo switchgrass. Lignin (r = 0.69; P < 0.0001 and r = 0.98; P < 0.0001) and cellulose (r = 0.60; P < 0.0001 and r = 0.92; P < 0.0001) concentration was positively correlated with ADF and NDF concentrations, respectively. Hemicellulose was positively correlated with only NDF (r = 0.53; P < 0.0001; Table 5).
3.6. Sugar Composition
Carbohydrate concentrations in switchgrass were different due to both genotype and N rate main effects (P < 0.05). Glucose, xylose and pentose represent the major components of cell-wall carbohydrates. Glucose concentration was similar between the two experimental lines, which were greater than Alamo and Cave-in-Rock (Table 6). Genotype NF/GA992 had the greatest arabinose, mannose and xylose concentrations but also had less starch and sucrose concentrations among all genotypes (Table 6). Arabinose concentration was different among all genotypes (P < 0.0001). Arabinose, pentose and xylose were the only sugars that responded to N application with their concentrations decreasing with increasing N rate (Table 6). Ethanol concentration, fructose and galactose were inversely correlated to ADF (Table 5). As lignin concentration increased, sugar concentrations decreased except for glucose.
![]()
Table 5. Significant correlation coefficients (P < 0.05, Pearson’s correlation test) among switchgrass biomass yield, cell wall properties and ethanol yield of switchgrass averaged across the 2008 and 2009 growing seasons at Brown Loam Branch Experiment Station, Raymond, MS.
†ADF = acid detergent fiber; NDF = neutral detergent fiber; Lig = lignin; Cell = cellulose; Hemi = hemicellulose; Ara.= arabinose; ETOH = ethanol g−1 dry forage; Fru. = fructose; Gal. = galactose; Glu. = glucose; Pent. = pentose; Sta. = starch; Xyl. = xylose; TEY is the total ethanol yield from SSF; TEP is the total ethanol production per hectare from SSF; TTEY is the total theoretical ethanol yield (100% conversion) and TTEP is the total theoretical ethanol yield production per hectare (100% conversion).
![]()
Table 6. Composition of switchgrass genotypes averaged across the 2008 and 2009 growing seasons at Brown Loam Branch Experiment Station, Raymond, MS.
†Ara. = arabinose, ETOH = ethanol g−1 dry forage; Fru. = fructose; Gal. = galactose; Glu. = glucose; Glcs. = soluble glucose; Man. = mannose; Pent. = pentose; Sta. = starch; Suc. = sucrose and Xyl. = xylose; ‡Within column, means followed by the same lowercase letters are not different using PDIFF in SAS (P > 0.05); §OPC, orthogonal polynomial contrast (L, linear; Q, quadratic; C, cubic); *, **, ***, significant at the 0.05, 0.01, and 0.001 levels, respectively; NS, not significant.
3.7. Ethanol Yield
There was a genotype (P = 0.0197) and N rate (P = 0.0161) main effect on TEY. Total ethanol yield was greatest in Alamo (165.8 L∙Mg−1) and averaged 162.0 L∙Mg−1 for the other three genotypes (Table 7). The TEY decreased linearly with N application rate (y = ?1.64x + 166.85, r2 = 0.89).
There was a year × genotype (P = 0.0465) and year × N rate (P = 0.0063) interaction effect on TEP. The year × genotype interaction is partly due to the lesser TEP in Cave-in-Rock in both years. The TEP also decreased linearly with N application rate in both years. The TEP is calculated from the biomass yield and therefore the trends in response to N application and genotype are similar to biomass yield trends. The TEP increase in 2009 was due mainly to the increase in the second year biomass yield.
Total ethanol yield was inversely related to lignin concentration and positively correlated with arabinose and ethanol concentration (Table 5). On the contrary, TEP was not correlated with the cell wall properties but was correlated to biomass yield (Table 5). Although, differences in biomass composition existed among the genotypes, biomass production was found to have a greater effect on ethanol yield than biomass composition. The selection of genotypes for a specific eco-edaphic zone can therefore be based primarily on biomass production [13] since differences in cell wall composition are not large enough to have a substantial effect on the biomass quality [16] .
3.8. Theoretical Ethanol Yield
There was a year × genotype interaction effect on TTEY (P = 0.0422). In 2008, TTEY was similar across all genotypes but in 2009, Alamo had less TTEY than Cave-in-Rock, although neither was different from the two breeding lines (Table 7). Lack of N application effect on TTEY is supported by previous studies [16] . The TTEP of Alamo and the experimental lines averaged 4.1 kL∙ha−1 in 2008 and 6.8 kL∙ha−1 in 2009 while Cave- in-Rock was 2.0 and 2.9 kL∙ha−1 in 2008 and 2009 respectively (Table 7). Across genotypes, TTEP in 2009 was 61% greater than 2008 and was correlated with biomass yield. There was an N rate × year interaction (P = 0.0031) effect on TTEP. During 2008, TTEP response to N application was linear but in 2009, the response was quadratic (Table 7). Total theoretical ethanol yield was positively correlated with ADF (r = 0.82; P < 0.001), NDF (r = 0.92; P < 0.001), cellulose (r = 0.84; P < 0.001), hemicellulose (r = 0.57; P < 0.001), glucose (r = 0.96; P < 0.001), pentose (r = 0.90; P < 0.001), and xylose (r = 0.96; P < 0.001) concentrations. Similar to TEP, the
![]()
Table 7. Estimated and theoretical ethanol yield of switchgrass genotypes during the 2008 and 2009 growing seasons at Brown Loam Branch Experiment Station, Raymond, MS.
†TEY is the total ethanol yield from SSF, TEP is the total ethanol production per hectare from SSF, TTEY is the total theoretical ethanol yield (100% conversion) and TTEP is the total theoretical ethanol yield production per hectare (100% conversion); ‡Within column, means followed by the same lowercase letters, and within rows, means followed by the same uppercase letters are not different using PDIFF in SAS (P > 0.05); §OPC, orthogonal polynomial contrast (L, linear; Q, quadratic; C, cubic); *, **, ***, significant at the 0.05, 0.01, and 0.001 levels, respectively; NS, not significant.
TTEP was correlated with biomass yield but not biomass composition (Table 5). Total ethanol yield from SSF and all biomass sugars did not correlate because of the differences in the methods of estimation. On the other hand, TEP from SSF and TTEP from all biomass sugars were correlated (r = 0.99) and both were also correlated with biomass yield (r > 0.99). Across all treatment combinations, TEP from SSF and all biomass sugars was 1.9 kL and 4.9 kL, respectively. Total theoretical ethanol production from all biomass sugars therefore increased by factor of 2.5 relative to TEP from SSF.
These data suggests that there is potential to increase the ethanol output from lignocellulosic biomass. Current methods of conversion are affected by cell wall recalcitrance and therefore do not fully liberate the cellulose and hemicellulose from the lignin complex for chemical, microbial or enzymatic depolymerization [25] [26] . Moreover, current methods are not cost-effective to facilitate commercial biomass to ethanol conversion [26] [27] . The ethanol yield potential difference in our study is 231 (141%) and 260 L∙Mg−1 (160%) in 2008 and 2009, respectively. Developing novel pretreatment methods to maximize the cellulose accessibility to cellulase enzymes may lead to improved biofuel processing and lower the cost of these biomass-based fuels.
4. Conclusion
Nitrogen fertilizer application will be necessary to optimize and maintain feedstock yield and persistence in switchgrass production systems. Genotype and N application rate had an effect on biomass yield and nutrient removal. Nutrient removal was related to biomass yield and cultivar-inherent biomass production capacities and their responses to N rather than tissue nutrient concentration. Nitrogen use efficiency and ANR decreased with increased N application. Application rates above 80 kg N ha−1 reduced NUE and ANR and were likely to result in increased N leaching and volatilization. Biomass production, nutrient removal, NUE and ANR for Cave-in- Rock were less than Alamo and the two experimental lines derived from Alamo. Although compositional differences exist among genotypes, there is no effect of N application on cell wall properties and several sugars. The differences in biomass yield had greater importance than the differences in biomass quality. Ethanol yield was more closely related to biomass yield than the small differences in compositional traits among the genotypes. The differences between estimated and theoretical ethanol yield provide opportunities for improving the pretreatment techniques to maximize the ethanol output. Findings from this research can further aid in cultivar selection, feedstock quality control, agronomic management, and policy decisions.
Acknowledgements
We thank Juan Solomon for assistance in field data collection and Dr. Rocky Lemus for assistance with NIRS analysis. This material is based upon work performed through the Sustainable Energy Research Center at Mississippi State University and is supported by the Department of Energy under Award Number DE- FG3606GO86025.
Disclaimer
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Abbreviations
ADF, acid detergent fiber;
ADL, acid detergent lignin;
ANR, apparent nitrogen recovery;
DM, dry matter;
ETOH, ethanol per gram dry forage;
NDF, neutral detergent fiber;
NIRS, near infrared spectroscopy;
NUE, nitrogen use efficiency;
OPC, orthogonal polynomial contrast;
SSF, simultaneous saccharification and fermentation;
TEP, theoretical ethanol production;
TEY, total ethanol production;
TTEP, total theoretical ethanol production;
TTEY, total theoretical ethanol yield.