Selection of Maize Genotypes with Tolerance to Osmotic Stress Associated with Salinity ()
Received 8 January 2016; accepted 19 February 2016; published 22 February 2016

1. Introduction
Saline soils are abiotic factors with great negative effect exerted on world agriculture [1] . The identification of crops tolerant would be an effective strategy to overcome the saline stress. Salinity affects some physiological and biochemical processes of the plants and reduces significantly the yield. Photosynthesis is the most important process affected by salinity; this may be due, among other things, to the closure of the stomata. Besides, it reduces the ability of the plant to use water, which causes a decrease in the rate of growth [2] - [4] .
A two-phase model to explain the inhibition of the growth under salinity conditions was proposes. The first phase is known like osmotic phase, where the decrease of the water potential of the soils is produced by the salt accumulation [5] .
Osmotic effects are observed rapidly after the salt application and continue during all the exhibition time determining the inhibition of the expansion and cellular division, as well as the stomata closure [3] [6] [7] .
The second stage is called an ionic phase where the inhibition is due to a rapid increase of the salt in the apoplast or the cytoplasm when the vacuoles cannot accumulate any more salt ions. The ionic effects cause an early senescence of the old leaves, with symptoms of toxicity (chlorosis and necrosis) [8] [9] .
In addition, the high concentration of Na+ affects the synthesis of proteins and enzyme activity [10] . The reduction of growth by salinity would be mainly due to the osmotic effects rather than the ionic [6] . However, it is also possible that ionic effects arise in the first phase and the osmotic effects persist in the second phase [11] [12] . Plants have developed three mechanisms to tolerate salt stress. The first one is that of tolerance to osmotic stress (related to the first phase of reduction of the growth) and the other two mechanisms (associated with the ionic stress) are the exclusion of Na+ from the leaves and the tolerance of the tissues [6] . In wheat and other cereals, it will appear that salinity tolerance is associated with the exclusion of Na+ [13] . Maize is considered as a crop moderately sensitive to salinity [14] and has been characterized as a plant that presents the exclusion of Na+ as a method of tolerance [15] . However, it is believed that the two other mechanisms will also be important in determining the tolerance [6] .
Osmotic stress reduces immediately the expansion of the roots and young leaves which determine a reduction in the size of the plant. This reduction can be attributed to a decrease in the rate of growth, including cell division and cell expansion, while the duration of growth will not be affected [7] . Osmotic phase could stay several hours or days before the Na+ concentration reaches toxic levels. In this way, daily measurements of the length of the young leaves would be a good indicator of osmotic stress. Plants tolerance to osmotic stress would be that keeps the rate of growth during the first days of exposure to salinity [6] . This response can be seen as an adaptive feature that reduces the loss of water by transpiration or as a reduction in stomatal efficiency by partial or total closure of the stomata which affects photosynthesis [7] . Thus the improvement of the tolerance to the osmotic stress could involve two opposing strategies. The first of these is to select plants with lower leaf area which will avoid water stress, and is associated with improvement of stomatal efficiency. While the second strategy is to select plants that keep the leaf area and the interception of light, which will be related to the improvement of the efficiency of absorption of water from the roots [7] .
The aims of this study were: (a) to assess the effects of salinity on growth and water economy traits in seedlings of maize, as a way to identify the presence of osmotic tolerance and b) to explore the presence of genetic variability for such a mechanism; with the purpose of identifying genotypes of contrasting behavior useful for gene effects’ studies and for breeding programs.
2. Materials and Methods
2.1. Plant Material
We selected 13 maize inbred lines (genotypes) that represent a wide range of racial origins, maturity and grain type (Table 1).
2.2. Hydroponic System
Seeds from the different genotypes were sterilized in the surface with 1% sodium hypochlorite solution for 5 minutes before experimentation, and then rinsed with distilled water. Pre-germinated caryopses were transferred to pots containing perlite. These pots were put in trays with 1/4 strength Hoagland’s solution. The full-strength nutrient solution had the following composition: in mol∙m−3, Ca(NO3)2, 2.5; KH2PO4, 0.1; K2SO4, 0.5;
![]()
Table 1. Food and Agriculture Organization of the United Nations (FAO) maturity (Short: minor than 500; Medium: between 500 to 700; Large: major than 700) and type and color grain (O: Orange, Y: Yellow, F: Flint; D: Dent) of the 13 evaluated genotypes.
MgSO4, 0.6; CaCl2, 5; in mmol∙m−3 H3BO4, I; MnSO4, 2; ZnSO4, 0.5; CuSO4, 0.3; NH4MO7O24, 0.005; Fe-EDTA, 200. Daily increments of 1/4 of concentration in the nutrient solution were made to reach the complete solution; the pH solution was maintained at 6.The solutions were renewed every three days.
2.3. Treatments
The experiment was carried out in a controlled environment room at 25˚C, with 16 h day length. A completely randomized design with three replicates was adopted. Each replicate was a plot with three plants (experimental unit).Three plots per accession were assigned to each treatment. Two treatments were used: 0 mM NaCl (as control) and 100 mM NaCl [16] [17] . The final concentration was reached by a gradual increment of 25 mM NaCl every day [18] . After 15 days of complete salinization, the seedlings were harvested.
2.4. Measurement
Leaf Growth Measurement after completing the salinity both the length and width of 4th and 6th leaf were measured every 2 days (4 measurements in total: Ar1 to Ar4). Also, the area of the blade on each measurement was estimated [19] .
Length Root (LR) measured in cm.
Shoot Dry Mass (SDM) and Root Dry Mass (RDM), were obtained after drying in an oven at 70˚C to constant weight.
Relative water content (RWC) was determined on cut leaves in pieces [20] . Applying the following formula:
 (1)
(1)
where FW = fresh weight, obtained immediately after the harvest; DW = dry weight, obtained by drying the sample in an oven until constant weight and TW = weight of turgor, determined then to rehydrate the bits of leaves for 2 hours.
Leaf Water Loss (LWL) The fresh weights of pieces of leaf were recorded (W1), then these pieces were left to evaporate under ambient conditions for 2 hours then turned to weigh (W2). The following formula was used:
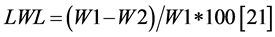 . (2)
. (2)
Stability of membrane (IE) was determined on the 6˚ leaf with the use of a conductivity meter (Consort C931) [21] . A piece of leaf was cut, weight and washed with distilled water, then these pieces were placed in a tube with 10 ml of distilled water and left to incubate at 10˚C in cooled incubator for a period of 24 hours [22] . After incubation, it was left to stabilize the sample to room temperature and the conductivity of the solution (M1) was measured. Then, the samples were autoclaved by 15 minutes to kill the tissue, were left at room temperature. The conductivity of solutions was again measured (M2). The stability index was obtained from the following formula:
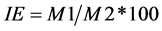 . (3)
. (3)
The morphological traits of growth (Ar1, Ar2, Ar3 and Ar4, SDM and RDM) were expressed in relative terms as a ratio between the measurements in salt over the same measurement in the control without salt, while the traits associated with the economy of water: RWC, LWL and IE were expressed in absolute terms.
Tolerance index was estimated for each trait that showed significant differences in the ANOVA. The variables expressed in relative values were divided by the best value obtained, in such a way that the best behavior in salt was corresponded with a value equal to 1 while the other values were less than 1. Summarized these values a tolerance index for each genotype was calculated.
2.5. Statistical Analysis
A DCA with an unequal number of repetitions was used. Data were subjected to analysis of variance and the Fisher’s least significant difference (LSD) was used for the comparison of means [23] . Numerical taxonomy techniques were applied to classify the genotypes across quantitative traits measured only in salt conditions [24] . A basic data matrix was standardized due to different measurement units of the variables. We performed a cluster analysis, based on two distance coefficients: Average taxonomic distance and Manhattan distance [25] . Subsequently the UPGMA linkage method was applied on both matrices, using NTSYS-pc program [26] . Cophenetic correlation coefficient was used to select the methodology to produce less distortion. Only the dendogram reached the highest cophenetic correlation coefficient value (CCC) was presented. The cut of the groups was performed in a subjective way, using a distance coefficient major to 1. A one way analysis of variance and comparison between means, using LSD test (p < 0.05), were applied to probe differences between clusters [23] . Principal component analysis (PCA) was performed to classify genotypes in a tri-dimensional graph and to identify the importance of the traits and the variance percentage explained by each one of the three first principal components [24] .
3. Results
The measured traits showed the presence of significant differences for genotypes, except for the character Ar1 that did not show significant differences (Table 2).
The application of LSD Test revealed the presence of differences in the behavior of the tested genotypes. Comparison for LR identified the genotypes: P21, AD3, Gaspe and AFE as those of major growth of root while the genotypes LP3, F2 and B73 showed a less radicular growth (Figure 1(a)). The frequencies distribution of the genotypes for the SDM and RDM showed a great similarity. In addition the genotypes WXEb, SC2 and AD3 presented the best behaviors and differ significantly from LP3 and SC75m (Figure 1(b) and Figure 1(c)). The measurement of the LWL and the RWC are simple, quick and easy ways to have an idea of the water status of the plant. The genotypes evaluated for LWL showed statistically different behavior (Table 3, Figure 1(d)). AFE, SC2, AD3 and SC75m genotypes had the smallest loss of water than LP3 and B73. For RWC, F564, Gaspé and SC75 genotypes had higher water content and differed statistically from P21, SC2, and WXEb with the lower water content (Figure 1(e)). The evaluated genotypes showed significant differences for IE. The AFE, F2 and SC75m genotypes were those who suffered most damage of membrane, while Mo17, AD3 and Gaspé presented the lowest one (Figure 1(f)).
Evaluation of the leaf area measured on the leaf 4th (Figure 2) showed that the 2nd measurement was the most affected by salinity, making values less than 1. P21, AD3, Mo17, Gaspe and F564 genotypes increased the range of growth along all measurements, while the remaining genotypes stabilized or decreased in the 4th measurement
![]()
Figure 1. Least significant difference (p < 0.05) for the comparison between maize genotypes exposed during two weeks to 0 mM (control) and 100 mM of NaCl doses. Relative’s values were calculated as the ratio between the value measured in salt and the value measured without salt, control. (a) Relative Length Root; (b) Relative shoot dry mass; (c) Relative root dry mass; (d) Leaf water loss (LWL); (e) Stability of membrane (IE).
![]()
Figure 2. Means and typical error of the relative leaf area measured on the 4th leaf (Area2, Area3 and Area4) recorded in maize genotypes stressed after four, six and eight days of exposure to 0 mM and 100 mM of NaCl doses. LSD test (p < 0.05) was separately calculated for each moment.
![]()
Table 2. Variance analysis of maize genotypes exposed during two weeks to 0 mM (control) and 100 mM of NaCl doses. Relative’s values were calculated as the ratio between the value measured in salt and the value measured without salt, control. LR: length root; SDM: shoot dry mass; RDM: root dry mass; Ar2, Ar3 and Ar4: 4th leaf area measured each two days.
ns: non-significant; *,**significant at p < 0.05 and p < 0.01, respectively.
![]()
Table 3. Two ways variance analysis of maize genotypes exposed during two week to 0 mM (control) and 100 mM of NaCl doses. Traits were measured in absolute values, LWL: leaf water loss; RWC: relative water content; IE: stability of membrane.
ns: non-significant; *,**significant at p = 0.05 and p = 0.01, respectively.
(8th day). AFE held a significantly lower relative growth rate at all moments. LP3 genotype showed a good rate of growth in the first measurements however it was that had greater loss of growth in the last measurement (Figure 2).
The salinity affects the rate of growth rather than growth time [7] . It means that the emergence of the 6th leaf would delay due to salinity. It can be seen in Figure 3 where the lines: Mo17, WXEb, SC75m, P21, LP3, and AFE delayed the appearance of the 6th leaf and this causes a significant reduction in growth associated with saline treatment. Besides, among these genotypes LP3 showed significant differences in growth for the treatments with and without salt.
Manhattan distance coefficient and UPGMA linked method grouped genotypes in 4 clusters (Figure 4) with a cophenetic correlation coefficient of 0.77, which would indicate an acceptable adjustment between the dendrogram and the true distances between genotypes. A LSD Test (using a significance level of p < 0.05) was performed to prove significant differences among the obtained clusters. Clusters didn’t show significant differences for IE and LR traits. Group I included genotypes higher values for Ar3 (mean: 1.05), Ar4 (mean: 1.085), SDM (mean: 1.08) and RDM (mean: 1.12). Contrary Group IV, conformed to AFE inbred line alone showed the lowest means, whatever It had no differences with cluster I and III for RDM and LWL, respectively. Thus, Clusters II and III showed mid values and non-significant differences with cluster I for Ar2, Ar3, Ar4 nor with the cluster IV for SDM and RDM.
Principal Component method identified the first three components which explained the 77.6% of the variability present in the original variables. The genotypes were classified as the dendrogram. PC1 explained the 42.4% of the variability with high and positive correlations with Ar4, SDM, Ar3, Ar2 and PC2 explained the 18.7% of the total variability and showed moderate and positive correlations with RWC, LR and LWL. PC3 explained 16% of the variability with positive correlations with IE and RDM but negative correlation with RWC (Figure 5).
The tolerance index selected two extremely genotypes, SC2 and AFE, which presented values of 8.67 y 6.7, respectively (Table 4).
4. Discussion
Osmotic stress causes a rapid inhibition in the expansion of the young leaves and reduced stomatal conductance
![]()
Figure 3. 6th leaf length for each maize genotype considering Control (0 mM NaCl) and Saline (100 mM NaCl) treatment. The leaf length was measured each two days (1, 2, 3 and 4 represent the 6th, 8th, 10th and 12th day from the complete salinization, respectively). Each value is the mean of 12 replicates.
![]()
Table 4. Tolerance index for 13 inbreed lines sorted in descending order.
![]()
Figure 4. Classification of maize genotypes growing in salt conditions (100 mM NaCl): Dendrogram build with the Manhattan distance y UPGMA and using absolute means values of the 9 traits: length root; shoot dry mass; root dry mass; Ar2, Ar3 and Ar4: 4th leaf area measured each two days; relative water content; leaf water loss; stability of membrane. Cophenetic Correlation Coefficient: 0.77.
![]()
Figure 5. Maize genotype exposed during two weeks 100mM of NaCl grouped using Principal Component Analysis. Traits were: LR: length root; SDM: shoot dry mass; RDM: root dry mass; Ar2, Ar3 and Ar4: 4th leaf area. RWC: relative water content; LWL: leaf water loss; IE: stability of membrane. Three dimensional graphics expressed the 77.6% of total variability. Numbers inside the squared represents eigenvector’s coefficients for each trait.
of mature leaves. Evaluation of the 4th leaf growth and the time of emergence and final development of the 6th, were used to measure osmotic stress. It can be assumed that the reduction in growth after 7 days of exposure in salt just might be more osmotic effects that the accumulation of Na+ (ionic effect). The decrease in leaf area could be considered as the first response of glycophytes plants in salinity. This behavior would be a way of avoiding excessive water loss by transpiration, although on the other hand, the reduction in leaf area could have adverse effects on gas exchange and photosynthesis [6] [27] .
The genotypes showed significant differences in reducing the growth of the 4th leaf [12] [17] [28] . The different degrees of reduction could be signaling the presence of genetic variability among the genotypes for osmotic stress associated with salinity. Also it could be seen, that the 4th leaf elongation rate presented differences between genotypes. The genotypes: WXEb, LP3, AFE, SC75m and F2 did not show exponential growth in the last measurement. It appears that growth stagnates or is lower than that observed in controls. This would be pointing out that these genotypes in salinity have a greater reduction in growth and consequently would be sensitive to the salinity. In addition, the delay in the appearance of the 6th could be another evidence of their sensitive behavior to salt [6] .
Salinity also produced significant reductions among genotypes for SDM and RDM; in consequence the most affected inbreed would be susceptible to salinity while the tolerant ones would suffer a smaller reduction [12] [17] [29] . Tolerance tests of short-term guarantee that the responses observed are due largely to the osmotic stress, because still there are not sufficient accumulation of Na+ as to produce damage [5] [11] .
The decrease in the rate of growth of leaves, after salt exposure may be due to osmotic effects caused by cumulative salts surrounding the roots. This determines a loss of water and turgidity of the cells. In this regard, assessments of the plant water status could help in the selection of the genotypes. For this purpose, LWL and RWC can be very useful. LWL is an indicator of “residual transpiration” that are associated with the loss of water through the cuticle tissue with a minimum stomatal opening [30] . The RWC is strongly related to the cell volume and can reflect the leaf water balance and the transpiration rate. One of the first symptoms of the deficiency of water in the tissues is the decrease of the RWC. However, there was no significant difference among the genotypes for the character [31] ; instead, [32] detected differences. Due to this ambiguous behavior, RWC not be a reliable trait to identify tolerant genotypes.
The loss of the integrity of the cell membranes and the increase of permeability are the first damage caused by salt stress. Therefore, the cellular ability to control the movement of ions would give an idea of the degree of tissue damage. The character IE was used successfully in the selection of salinity tolerance in wheat [33] [34] . Changes in the composition and structure of the cell membrane by salinity stress would show differences between tolerant and sensitive species. Our results also showed differences in the behavior of the genotypes for IE [22] .
The information obtained from the application of Principal component analysis, cluster analysis and indexes of tolerance allowed the identification of genotypes according to their behavior as tolerant or susceptible. The PCA identified 4 groups. The genotypes SC2, AD3, WXEb and P21 were located in the first group. This group included individuals who have a great development of the stem (high SDM and Ar2, Ar3 and Ar4) and root (high RDM). However, these genotypes showed differences in the water status. In this group highlighted the genotype SC2 that showed the greatest shoot growth; although its water content (RWC) was low which would indicate that used efficiently absorbed water. The second group was integrated by genotypes Gaspé, SC75m, F564, F2 and Mo17, which were characterized for showing a moderate growth of shoot and root, although differ in water content. The third group was composed by B73, LP3 and SC75 which presented a strong reduction of the shoot growth and water content. In the last group ranked the AFE genotype that presented lower shoot growth but higher root length, and a greater loss of water by cuticular transpiration and high damage to cell membranes (IE higher) (Figure 4).
Tolerance index allowed to classify genotypes, thus SC2 and AD3 lines were which reached highest value of the index and therefore would be tolerant lines, while AF3 and LP3 had a low index and were seen as sensible.
5. Conclusions
We studied the effects of salinity on growth and water economy traits in seedlings of maize, to explore the presence of genetic variability for osmotic tolerance mechanism; with the purpose of identifying genotypes of contrasting behavior useful for gene effects’ studies and for breeding programs. We employed three different methodologies for the screening: Cluster Analysis, Principal Component Analysis and a Tolerance Index. Whichever the methodology used for genotypes classification had similar results and therefore they could indistinctly be used as a selection tool.
Thus, the selected genotypes as tolerant were SC2 and AD3 while AFE and LP3 were identified as susceptible. These inbreed lines with contrasting behavior could be used as putative parents to obtain segregating populations (Fn or RILS) capable of being applied to genetic studies such as the identification of quantitative traits loci (QTLs analysis) associated to osmotic stress.
NOTES
![]()
*Corresponding author.