Investigation of the Stability of Oxadiasole and Their Analogs Using Quantum Mechanics Computation ()
Received 20 November 2015; accepted 10 January 2016; published 13 January 2016

1. Introduction
Most components consisted of five-member heterocyclic rings have special chemical properties and are used for multipurpose biological activities [1] . Oxadiazole is an aromatic heterocyclic with C2H2N2O formula. It has 4 isomers including 1,2,4-Oxadiasole, 1,2,5-Oxadiasole and 1,3,4-Oxadiasole as its well-known isomers and 1,2,3- Oxadiasole as its only unstable isomer [2] [3] (a diazoketone tautomer). Stable Oxadiasole exists in an extensive range of drugs including Raltegravir, Butalamine, fasiplon, oxolamine, pleconaril [4] - [7] . Thiadiasoles (C2H2N2S) are categorized based on their pharmaceutical effects and the emphasis is on their versatility in pharmaceutical chemistry [8] [9] . Unique properties of Thiadiasoles are also a special subject that is investigated with regards to their effects on various activities. Thiadiasole is one of thebiological iso-esters of Oxadiasoles which are considered for their pharmaceutical properties [10] [11] . They are five-member hetrocyclic components that contain Nitrogen, oxygen, and sulfur atoms. They’ve served as oxidation inhibitors, cyanine dyes, metal chelating agents and metal corrosion agents. Thiadiasoles which had been tested against various diseases successfully were important to pharmaceutical chemistry because of their various applications [2] [12] .
Oxidiasole is an important heterocyclic ring consisted of two Nitrogens and one Oxygen in a five-membered ring. Oxidiasoles have different analogs based on the placement of Oxygen and Nitrogen atoms in the ring. The aromatic properties of these analogs along with Thiadiasoles derivatives and also these properties’ effects on the stability of their different varieties are discussed here. In Figure 1, different Oxidiasoles derivatives are illustrated.
Oxidiasoles are usually employed for designing bioactive or affective agents on organisms (entities). This interesting group of components have various biological activities such as antibacterial, anti-inflammatory, anti- tuberculosis, anticonvulsants and etc. [13] [14] .
In this study, effective factors of different Oxidiasoles’ isomers on component’s structural stability are investigated, based on B3LYP/6-311+Gtheoretical level** and nuclear magnetic resonance (NMR) [15] - [19] .
2. Methods
This study was conducted in several gas phases. First, the isomers’ structures were optimized using semi-em- pirical quantum mechanics method. Then, the required Z-Matrix was prepared for using in Gaussian 03 program. Next, Stereo-electron thermodynamic parameters were computed to obtain final optimized structures as well as to calculate electron energies using GIAO/B3LYP/6-311+G level** that is one of the high levels abinithio quantum mechanics available in Gaussian program [20] [21] .
Furthermore, according to relative density theory, several total chemical reactivities descriptorssuch as hardness index, chemical potential, softness, electronegativity and electro-philicity were introduced [22] - [24] . The results show that molecular stability depends on hardness. Pauling introduced electronegativity as a force from one atom to the other to maintain and capture its electrons. Equations 1 to 3 show hardness (η), chemical potential (μ), and electronegativity (X) formula, respectively.
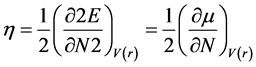 (1)
(1)
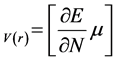 (2)
(2)
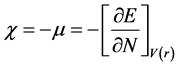 (3)
(3)
Parr et al. investigated electrophilicity as a measurement unit for decreasing energy that considered the maximum of electrons exchanged between electron donor and electron acceptor [25] - [27] . They defined electrophilicity in the following equation:
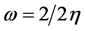 (4)
(4)
If one of two molecules reacting together acts as an electrophil, that molecule has larger electrophilicity index (electron acceptor). This new reactivity index measures the stability of energy when it captures an extra electron charge (ΔN) from surrounding. Electrophilicity is a reactivity descriptor that accepts some natural Electrophilicity in relative scale. Recently, it’s proven that this quantity can be used to assess the toxicity of various pollutants based on their reactivity and selected place [25] [26] [28] [29] .
3. Result and Discussion
In this section, the relation between aromaticity and stability of various Oxidiasole’s analogs in Figure 1 is investigated by different parameters including length of bonds, GΔ, hardness, softness, as well as NICS and ASE
computations. Furthermore, by comparing data and charts resulted from quantum mechanics computation it is tried to justify the stability of required molecules and their relation with aromaticity.
In analogs containing Oxygen atom i.e., oxadiasole components 1 to 4, it is founded that the most stability is for component number 4, which has the smallest amount of free Gibs energy. Stability level of component number 1.
Component number 2 and component number 3 are 21.86, 8.64, and 40.61 kcal/mol, respectively. According to the results, in analogs under investigation the stability level decreases as follows:
4 > 2 > 1 > 3
So that component number 4 is the most stable and component number 3 the least stable component (Table 1).
3.1. Nucleus-Independent Chemical Shift (NICS)
One of the factors which can affect the stability of a component is its aromaticity properties. In order to assess the aromatic property of these components, nucleus-independent chemical shift (NICS) method was used.
All computations are performed in GIAO-B3LYP/6-311+G** theoretical level.
The amounts calculated in NICS for each component can be negative or positive. Negative amounts state component’s aromaticity, while positive amounts state anti-aromaticity of a component.
All components investigated in this study are aromatic because they all have negative NICS amounts.
For all components, NICS amounts in molecular center (NICS (0)) and in different distances on molecular surface had been calculated.
Results of NICS computations show the smallest amount for component number 4. According to these computations, the highest NICS amount belongs to component number 3 (Table 2).
3.2. Aromatic Stability Energy (AES) [1]
Aromatic stability energy is one of the methods used for studying aromatic properties of components. For this purpose, a series of isodesmic reactions are considered and aromatic stability energy (AES) amount is measured by Equation (5) [30]
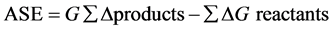 (5)
(5)
Results of this calculation are arranged in Table 3.
The more the amount of aromatic stability energy (ASE) is, the more stable is the component. Energic computations show that component number 4 is more stable, but ASE results cannot justify component number 4’s stability. In Table 3, it can be observed that component number 4 is the most stable component with highest amount of hardness.
The more is the hardness of the molecule, the more stable it is. Considering the results in Table 4 the highest amount of hardness, 0.1327, belongs to component number 4. And the least hardness among the components belongs to component number 1, with the amount of 0.1178. The calculated results for molecule’s hardness can justify component 4’s stability compared to components 1, 2 and 3.
As can be noticed in Table 4 the amounts of molecules’ softness also decreased from components number 1 to 4 which can also confirm the stability of component 4 compared to other components.
3.3. Length of Bond
One of the most effective parameters that can justify stability is the length of bonds in a component.
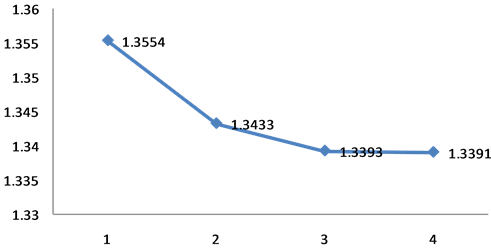
Chart 1. Measured average amounts forlength of bonds of com- ponents 1 to 4 from chem 3d.
![]()
Table 1. Calculated ∆G amounts for components 1 to 4, in kcal/mol on theoretical level GIAO-B3LYP/6-311+G**.
![]()
Table 2. Amounts results obtained in different distances for components 1 to 4 in GIAO-B3LYP/6-311+G** theoretical level.
![]()
Table 3. Calculated AES amounts for components 1 to 4, in kcal/mol on theoretical level GIAO-B3LYP/6-311+G**.
![]()
Table 4. Amounts of electronegativity (X), hardness (η), softness (S) calculated in electron volt (ev).
In this study, the length of bonds’ average parameter is measured and specified for each component and the results are demonstrated in Chart 1.
According to the results in this step component number 4 which is known as the most stable component has the least amount of length bonds, which is 1.3392 angstrom (Chart 1).
Therefore, the stability of component number 4 (Oxidiasole-1,3,4) can be justified based on the length of bonds.
4. Conclusion
In components containing Oxygen, i.e. oxadiasoles 1 to 4, Component 4 is the most stable. This can be justified by the calculation of energy (ΔG), average of length of bonds, as well as hardness and softness; while NICS and ASE computations don’t explain it.