Mechanosynthesis as a Simple Method to Obtain a Magnetic Composite (Activated Carbon/Fe3O4) for Hyperthermia Treatment ()
Received 18 November 2015; accepted 3 January 2016; published 6 January 2016

1. Introduction
Magnetic nanoparticles offer some attractive possibilities in biomedicine. Firstly, they have controllable sizes ranging from a few nanometers up to ten nanometers, which place them at dimensions that are smaller than or comparable to different human body components. Secondly, the nanoparticles obey Coulomb’s law, and can be manipulated by an external magnetic field gradient. Thirdly, the magnetic nanoparticles can be made to resonantly respond to a time-varying magnetic field, with advantageous results related to the transfer of energy from the exciting field to the nanoparticle [1] .
This last feature makes the magnetic nanoparticles able to heat up leading to their potential use as hyperthermia agents. Magnetic hyperthermia has recently attracted significant attention as a safe method for cancer therapy. It can increase the temperature in tumors to 41˚C - 46˚C, thereby killing the tumor cells with minimum damage to normal tissue [2] .
Nano-magnetic particles with tailored surface chemistry have been widely used experimentally for numerous in vivo applications such as hyperthermia, magnetic resonance imaging (MRI) contrast agent, tissue repair, immunoassay, drug delivery, and cell separation [3] . The concept of magnetically mediated heating of iron-oxide nanoparticles is gaining increasing attention as a potential new cancer treatment [4] . The heating of oxide magnetic materials with low electrical conductivity in an external alternating magnetic field is due to loss processes during the reorientation of the magnetization [5] .
Among various magnetic nanoparticles, magnetite (Fe3O4) has been considered suitable due to its biocompatibility, ease of synthesis and heating properties [6] . Iron oxide nanoparticles have been synthesized by hydrothermal method [7] , hydrolysis [8] , sol-gel assisted electrospinning [9] , vapor-liquid-solid growth [10] , a solvothermal synthesis [11] , thermolysis [12] , a wet chemical process [13] , flame synthesis [14] , etc. However, co- precipitation is often employed because nanoparticles with uniform phase can be obtained and the synthesis processes such as reaction, washing and solid-liquid separation are simple [15] .
Moreover, the use of different matrix such polymers [16] - [19] and ceramics materials [20] - [24] to transport the nanoparticles into the body has been researched. The ability to manipulate/bind individual molecules at nanoscale has provided ample opportunity for new therapeutic and diagnostic applications. In this way, nanocomposites can be obtained or it may be embedded in biocompatible materials to impart new functionalities. Activated carbon is a good choice as a coating, due to its high surface area and known adsorption-desorption properties for many molecules including peptides, proteins and drugs [25] . The magnetic materials in activated carbon nanocomposites are better to be higher saturation magnetization (Ms) and less concentration for keeping considerably good adsorption performance [26] .
The aim of this work was to synthesize a magnetic composite using activated carbon with magnetite, so that being within the human body and contacting with an external magnetic field, is viable for its use for cancer treatment by magnetic hyperthermia technique.
2. Experimental
2.1. Activated Carbon Oxidation
The procedure was followed according to established by Rangel-Mendez and Streat in 2001 [27] . 5 g of activated carbon (AC) was placed in a three-necked flask with a solution of HNO3 with deionized water (50:50) and this was brought to a controlled temperature of 85˚C for 3 h. After the respective time, three-necked flask was removed and placed in an ice bath to prevent oxidation further progress. The nitric acid excess was removed and the activated carbon was washed with deionized water. Finally, the activated carbon was dried in an oven at a temperature of 80˚C for 24 h.
2.2. Obtention of Fe3O4 Nanoparticles
For the synthesis of this ferrite, FeCl2・4H2O and FeCl3・6H2O (molar ratio 1:2) were mixed in 50 mL of deionized water. On the other hand, in a ball flask were placed 150 mL of deionized water and heated at 70˚C at 1000 rpm using a mechanical stirrer.
As the temperature was reached, 50 ml of concentrated ammonium hydroxide (pH 9.8) were added in order to facilitate a basic medium in the solution with low stirring and heating to a temperature of 70˚C. Subsequently, mixture of iron chloride solution was added dropwise and left under constant stirring (5000 rpm) for half an hour.
Once the time has expired, the precipitate obtained was washed with 2 L of deionized water to remove excess chloride and allowed to dry at room temperature for 3 days. Finally, the product obtained was washed with 1 L of water and 250 mL of ethanol and allowed to dry at room temperature.
2.3. AC/Fe3O4 Composite Mechanosynthesis
Once obtained magnetite and the oxidized activated carbon, a carbon-magnetite composite (AC/Fe3O4) was obtained by mechanosynthesis technique. This procedure took place on the FRITSCH planetary mill brand, model Pulverisette 6. A certain amount of activated carbon and ferrite were added to the container and placed in agate mill at 400 rpm for 3 h in order to obtain a composite of ferrite and activated carbon 60%/40% respectively, due to this ratio showed the best heating properties obtained in preliminary tests, since the ferrite mediate the heating capacity. Finally, the product was washed with deionized water and allowed to dry at room temperature.
2.4. Materials Characterization
The magnetite nanoparticles and the composite AC/Fe3O4 were analyzed by X-ray diffraction (XRD) (Siemens Mod. D-5000). The magnetic properties of the samples were measured with a SQUID Quantum Design magnetometer (VSM) in applied fields from −12.5 to 12.5 KOe. The particle size and shape were studied by field emission scanning electron microscopy (SEM) (JOEL JSM-7401F) and energy dispersive X-ray (EDX) techniques. FT-IR spectra of the materials have been taken by a Perkin Elmer FTIR, model Spectrometer Frontier.
2.5. Heating Capacity
This technique was performed to determine whether the particles had the ability to generate heat. These tests consisted of placing certain concentration of sample, in a vial with 2 mL of water, which was stirred with a vortex. These vials were carried to an equipment of magnetic induction in solid state, which was programmed using a magnetic field (10.2 kA/m and frequency 200 kHz), during 15 minutes.
2.6. In Vitro Hemolysis Assay
In order to determine the biocompatibility of activated carbon and AC/Fe3O4 composite with human erythrocytes, hemolysis tests were performed. The hemolysis test was performed using human whole blood from healthy non-smoking donors, following the proper guidelines for studies using human specimens. The procedure was conducted as reported by Muzquiz-Ramos et al. in 2014 [28] . A certain amount of activated carbon and composite (AC/Fe3O4) were contacted with 150 µL of human blooderythrocytes in 1850 µL of Alsever’s solution (dextrose 0.116 M, NaCl 0.071 M, sodium citrate 0.027 M and citric acid 0.002 M, pH 6.4) in order to obtain concentrations of 0.5 mg/mL, 2 mg/mL and 3 mg/mL. The hemolysis percent was obtained by the Equation (1).
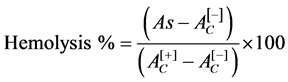 (1)
(1)
where:
As: Sample absorbance;
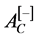 : Negative control absorbance (erythrocytes/Alsever’s solution);
: Negative control absorbance (erythrocytes/Alsever’s solution);
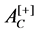 : Positive control absorbance (erythrocytes/deionized water).
: Positive control absorbance (erythrocytes/deionized water).
3. Results and Discussions
3.1. Structural Properties
XRD patterns of magnetite and the AC/Fe3O4 composite are shown in Figure 1. The diffraction peaks of the synthesized magnetite in Figure 1(a) can be perfectly indexed to the inverse spinel structure (JCPDS card no. 019-0629), and no characteristic peaks of impurities are detected in the XRD pattern, implying that the formation of the single phase spinel. As shown in Figure 1(b), for AC/Fe3O4 composite, could observe the most intense
![]()
Figure 1. XRD patterns of synthesized magnetite (a) and AC/Fe3O4 composite (b); and the reference data for Fe3O4 of the JCPDS file No. 16-0629.
peaks of magnetite. However, it has also been shown that activated carbon has a very broad peak between 40˚ and 60˚, peak that can be observed slightly in the composite XRD pattern. The disappearance of the other peaks may be due to overlap of signals by the amorphous structure of graphite [29] .
Furthermore, the crystallite size values were calculated by the Scherrer’s equation, taking the most intense peak and the Gaussian model. The crystallite size of the synthesized magnetite is 10.9 nm. Determining the particle size is very useful because, to use a material in magnetic hyperthermia, it must have a size between 9 and 15 nm. It has been shown that the spherical type nano-sized particles have higher diffusion speed, increasing the concentration of nanoparticles in the center of a blood vessel, thus limiting interaction of the nanoparticles with endothelial cells and prolong circulation time of the nanoparticles in the blood [30] .
The morphology of the AC/Fe3O4 composite was investigated by SEM observations and EDX analysis. As shown in Figure 2(a), EDX analysis indicates presence of phosphorus in activated carbon surface related to phosphates due to the activation process. Furthermore, in Figure 2(b), it can be seen the composite AC/Fe3O4 micrograph were Fe3O4 particles seems to be deposited on activated carbon surface. Also the EDX analysis indicates a decrease in the amount of C atoms on the composite, in comparison with the activated carbon and moreover the presence of Fe atoms.
In addition, Figure 3 shown the nanoparticles deposited in activated carbon surface, which have a size between 8 and 22 nm. These results are comparable to those obtained by the Scherrer’s equation of crystallite size using the diffraction data.
3.2. Magnetic Properties
The magnetic properties of the samples were measured in a SQUID Quantum Design magnetometer in applied fields from −12.5 to 12.5 KOe. Figure 4 shows the hysteresis loops of the synthesized magnetite (a) and AC/Fe3O4 composite (b). Both hysteresis loops exhibit a superparamagnetic behavior, however the results of saturation magnetization, remanent magnetization and coercivity are summarized in Table 1. Coercivity data are of great importance, as these show superparamagnetic behavior of the materials when coercivity values are near 0. In case of the synthesized magnetite coercivity value is 10.28 Oe and for AC/Fe3O4 composite coercivity value is 8.92 Oe. This coercivity values indicates that both samples have a superparamagnetic behavior.
Moreover, the saturation magnetization of AC/Fe3O4 composite decreases in comparison of the synthesized magnetite due to the presence of activated carbon, which has no magnetic properties. These results are consistent with those expected due to the presence of activated carbon, which does not show a magnetic behavior. Mendes et al. in 2014, attribute this difference in saturation magnetization due to the presence of a non-magnetic phase, for example an organic diamagnetic material as the activated carbon [31] .
3.3. Chemical Properties
In order to determine the bonds formed between AC and Fe3O4 in the mechanosynthesis process, infrared
![]()
Figure 2. SEM micrographs and EDX spectrum. (a) AC and (b) AC/Fe3O4 composite.
![]()
Figure 3. SEM micrographand magnetite particles measurement in AC/Fe3O4 composite (300000×).
spectroscopy tests were performed. FT-IR spectra of oxidized activated carbon (AC) and AC/Fe3O4 composite are shown in Figure 5. When activated carbon is contacted with an oxidizing agent such as nitric acid, it has been determined that the concentration of functional groups is increased [32] . Furthermore, also in Figure 4 it is possible to observe FT-IR spectra of AC/Fe3O4 composite in which there is clearly a decrease in the intensity of the bands between 1500 cm−1 and 1700 cm−1 and disappearance of OH− band (between 3500 cm−1 to 3000 cm−1), relative to AC precursor. This may indicate that magnetite is being bound to these functional groups in mechanosynthesis process due to the high energy formed in the milling. It is also clear oxygen-metal bond, since a
![]()
Figure 4. Hysteresis loops of the synthesized magnetite (a) and AC/Fe3O4 composite (b).
![]()
Figure 5. FT-IR spectra of activated carbon (AC) and activated carbon/magnetite composite (AC/Fe3O4).
![]()
Table 1. Magnetic properties of the synthesized samples.
band at 773 cm−1 is presented.
Moreover, the main functional groups that can be observed in the FT-IR spectra are summarized in Table 2, which for activated carbon principal bands attribution are phenolics and carboxylic acid groups, formed by the oxidation process in the material surface. For AC/Fe3O4 composite is more clearly detailed the disappearance of phenolic groups corresponding to bands and some kind of carboxylic acid bond, due to the bond formed between this oxygenated groups and the magnetite.
3.4. Heating Capacity
Furthermore, the heating ability of the composite was also tested. Analysis for different masses of AC/Fe3O4 composite (16 mg, 18 mg and 20 mg) were performed. Heating curves are presented in Figure 6, where the greater the amount of mass greater heating capacity was observed. However, for the mass of 20 mg, this composite managed to generate a temperature of 48.7˚C, temperature at which cell damage occurs known as heat ablation. According to the heating curve, 18 mg of the AC/Fe3O4 composite is able to use in hyperthermia technique, since this mass generated a temperature of 45.6˚C in 15 minutes. The adequate temperature for hyperthermia treatment it’s above 46˚C [33] .
3.5. Biocompatibility Test
For determine if the materials were biocompatible to human erythrocytes, hemolysis assay were carried out. This test is important since the material can be applied directly to the bloodstream, so that in a given moment be in contact with the erythrocytes of a human blood. Figure 7 shows the hemolysis percent caused by the AC/Fe3O4 composite at different concentrations. Activated carbon hemolysis percent is also reported. Error bars represent the mean and standard deviation for six experiments. The results of hemolytic test (Figure 7) demonstrated that the HR of the samples were lower than 5%. According to ASTM F 756-08 (Standard Practice for Assessment of Hemolytic Properties of Materials) [34] , HR < 5% produced by any material could be considered as not hemolytic. According to these results, we can deduce that the activated carbon and the composite AC/Fe3O4 are not a hemolytic materials and is biocompatible with human blood erythrocytes.
![]()
Figure 6. Heating curves for different mass of the composite AC/Fe3O4.
![]()
Table 2. FT-IR spectra bands observed in the samples.
![]()
Figure 7. Hemolysis (%) caused by AC (lines) and AC/Fe3O4 (square).
4. Conclusion
Activated carbon/magnetite composite was obtained by a simple mechanosynthesis method (400 rpm, 3 h). A superparamagnetic behavior is observed in the composite AC/Fe3O4. Magnetite particles are on the surface of the activated carbon according the observed in the SEM images, with a particle size between 9 - 14 nm. The decrease in intensity of bands of oxygenated surface groups of activated carbon in the FT-IR spectra, may indicate that ferrites have been attached to these groups. Moreover, the composite AC/Fe3O4 demonstrated a heat generation of 45.6˚C under a low frequency magnetic field. Furthermore, hemolysis tests indicated that AC/Fe3O4 is a non-hemolytic material, since the hemolysis percent was under 5%. The results show that the composite might be used for cancer treatment by magnetic hyperthermia therapy.
Acknowledgements
Authors gratefully acknowledge CONACyT-México for the provision of J.C. Ríos-Hurtado scholarship (423185) and SEP (PROFOCIE 2014 project) for the financial support on this research. The authors also thank S.G. Solís- Rosales and J.A. Cepeda-Garza from CIQA for their valuable technical and professional assistance. Finally, authors appreciate the financial support of CGEPI UAdeC for publishing this article.
NOTES
![]()
*Corresponding author.