Assessment of Lindane and Atrazine Residues in Maize Produced in Ghana Using Gas Chromatography-Electron Capture Detector (GC-ECD) and Gas Chromatography-Mass Spectrometry (GC-MS) ()
1. Introduction
The importance of maize (Zea mays) in global food production cannot be overemphasized. Its cultivation is done by both commercial and peasant farmers [1] as it is ranked third most widely cultivated and consumed cereal falling behind rice and wheat [2] . It is used significantly as a basic element of animal feed; raw material for industry as well as a significant source of nutrients for man. In Ghana, it is a major staple food crop whose domestic demand is projected to increase at an annual rate of 2.6% [3] . Though produced predominantly by smallholder farmers under rain-fed conditions, production has increased steadily since 1965 [4] - [6] . Increasing population has also led to an increase in demand for maize and food from maize sources hence the extensive use of pesticides especially atrazine in its cultivation. It is estimated about 95% of the Ghanaian populace consumes food from maize sources [7] and about 75% of the total maize produced in Ghana is also used as food while the remaining find their way into poultry feed and food grain sales industries [8] .
Pesticides help improve crop yield, protect crop quality, reliability as well as reduce the cost of production. However, it has been established that pesticides could become detrimental to health if misused. Some of the negative effects of pesticides misuse include destruction of soil micro fauna and flora and undesirable residue accumulation in food crops [9] . The use of these chemicals over the years has led to the contamination of various environmental compartments such as soil, ground and surface water, air as well as numerous agricultural food products [10] .
Lindane is an Organochlorine chemical and the only active isomer of hexachlorocyclohexane [11] . It has been used over the years as both agricultural and pharmaceutical chemical. When released into the environment, it remains intact for exceptionally long periods, becomes widely distributed throughout the environment; accumulates in tissues of living organisms and toxic to both humans and animals [12] . Lindane has been used over the years in maize production to control stem borers and also on cocoa and vegetable farms [13] . It belongs to the class IIB group of insecticides; because it is moderately acutely toxic. The international trade of Lindane is restricted under the prio-informed consent of the Rotterdam convention [14] . Under the Stockholm convention, the agricultural use as well as production of Lindane was banned due to its toxicity [15] . Moreover it was classified in 1987 by International Agency for Research on Cancer (IARC) as a group 2B chemical; meaning it is a possible human carcinogen [16] . The principal areas Lindane affects in humans are the nervous system, liver and kidneys. A range of symptoms such as convulsions, headache, seizures and dizziness occur when exposed to large amounts of Lindane [17] [18] .
Atrazine is a widely used herbicide as it is the second largest selling pesticide in the world. The worldwide increase in maize and other cereal production could be attributed to the use of herbicides such as atrazine. Works that reveal agricultural loses of about 500 percent without herbicide use only highlight the significance of herbicides in modern management of weeds in the production of maize [19] . A paper published in 1999, revealed positive maize yields upon atrazine application [20] . In a similar work, it was reported that maize gave higher yields when weed management was done with herbicides as opposed to controlling weeds culturally [21] . It has also been reported that application of herbicides gave 65% - 90% weed control and 100% - 150% more maize yield [22] .
In Ghana, atrazine is the major herbicide used. In spite of its use in increasing maize yield through weed control, it has been found to reduce the ability to successfully reproduce [23] . It induces estrogen production while inhibiting production of testosterone; causing chemical castration [24] . Research has shown that, the level of pesticides in food in many developing countries including Ghana is escalating [25] . Most of the levels found in humans are related to current and past use of these chemicals. Earlier studies in Ghana indicated the presence of pesticide residues in meat, water, sediment, vegetables, fruits, fish and soil at locations unknown for pesticide usage [26] - [30] . The high application of pesticides has resulted in food contaminations [31] ; ingestion of which has been linked with health conditions. Contaminated foods are a major source of morbidity, mortality and increased risk of skin cancer, destruction of neurological cellular functions, chronic neurotoxicity, bladder and lung cancer even at very low concentrations [29] [32] . The high consumption of maize by Ghanaians has thus warranted the need to assess the levels of atrazine and lindane residues so as to correctly estimate human dietary exposure and also refine risk assessments.
2. Materials and Methods
2.1. Field Investigation
A field survey was conducted in nine out of ten regions in Ghana (Figure 1) between November 2014 and April 2015. Questionnaires were administered to 111 farmers and traders to assess the knowledge, practice and attitudes regarding safe use of Atrazine and Lindane, toxicity awareness and symptoms among the farmers and traders. Face-to-face interviews with farmers, farm workers, traders and field observations during farming activities were also employed in data acquisition. During the survey dry maize samples were purchased from farmers and traders, wrapped in zip-lock bags and transported to the laboratory for analysis. Maize samples were bought from Brong Ahafo region (30 samples), eastern region (20 samples), Ashanti region (15 samples), Central region (14 samples), Northern region (11 samples), Volta Region (15 samples), Upper West Region (8 samples), Upper East Region (5 samples) and Western Region (2 samples). These regions were selected based on maize production figures of at least 60,000 metric tonnes per annum [33] . These samples were composited into nine representative regional samples.
2.2. Sample Extraction
The QuEChERS method was employed in the extraction process where FOSS homogenizer 2096 was used to homogenize the maize samples to powdery form. 10 g of powdered sample was then weighed using a Mettler Toledo PG1003-S mass balance into a 50ml centrifuge tube. 10 ml each of cold deionised water and acetonitrile were added. The mixture was then vortexed for one minute using a Thermolyne maxi mix plus. A mixture of-
QuEChERS salts was then added {(i.e. 4 g anhydrous MgSO4 plus 1 g NaCl, 1 g TSCD (trisodium citrate dehydrate), 0.5 g DHS (disodium hydrogen citrate sesquihydrate)}. The mixture was vortexed for a further one minute and then centrifuged for five minutes at 3000 rpm.
2.3. Sample Clean-Up and Concentration
Dispersive SPE was the method used in purifying the sample extract. 6ml aliquot of the extract was then transferred into a PP (Polypropylene) centrifugation tube containing 150 mg PSA (primary and secondary amine exchange material) and 900 mg MgSO4. The mixture was vortexed for one minute and centrifuged for five minutes at 3000 rpm. 4 ml of the supernatant (clean extract) was transferred into a pear-shaped flask. 40 µl of 5% formic acid in acetonitrile (v/v) was then added to adjust the pH. The filtrate was then concentrated below 40˚C on rotary evaporator to dryness. 1ml ethyl acetate plus 20 µl of 1% polyethylene glycol was then used to re-dissolve dry concentrate. Sample was then transferred into a 2 ml standard opening auto sampler vials for quantification by GC-ECD and GC-MS.
2.4. Sample Analysis by Gas Chromatograph
All samples were analyzed in a gas chromatograph (Varian CP-3800 GC-ECD with CombiPALAutosampler and GC-MS). It was fitted with a Varian analytical column with 30 m + 10 m EZ Guard × 0.25 mm id fused silica capillary coated with VF-5 ms (0.25 micrometer film). Sample vials were interspersed with analytical standards of interest and placed on the Autosampler. The Atrazine and Organochlorine components in the samples were identified by comparing their retention times with those of the standards of these chemicals while quantification was based on comparison with calibration curves. The chromatograph’s conditions were as follows: injector mode, Splitless; injector temperature 270˚C; oven temperature programmed from 70˚C, held for 2 minutes to 180˚C at a rate of 25˚C/minute, then from 180˚C to the electron capture detector with temperature set at 300˚C. Nitrogen at a flow rate of 1 ml/min constant flow with a make-up of 29 ml/min was the carrier gas used.
2.5. Quality Control/Quality Assurance
Quality control and quality assurance as prescribed by the CODEX Alimentarius Committee were incorporated in the analytical scheme. Quality assurance measures applied in the laboratory included rigorous contamination control procedures, monitoring of blank levels of solvents, equipment and other materials, analysis of procedural blanks, recovery of spiked standards, monitoring of detector response and linearity. During extraction, blanks and duplicates were included in the analysis and re-calibration standards run frequently to check the integrity of the calibration curve. The quality of analytical methods was assessed by pesticide recovery experiments with sample extracts. Certified reference material 1941b for pesticides residue from NIST, USA and analyzed by GC-ECD/MS. Blank Samples known to have no detectable levels of the investigated pesticides were used for recovery test. The results of the study were not corrected for recoveries since all were within the normal acceptable range of 65% - 120% [34] [35] .
2.6. Health Risk Estimation
To assess the risk of pesticide usage to farmers and consumers, the guidelines for potential risk assessment drawn up by the US EPA were followed [36] . A reference dose (RfD) was used to evaluate the chronic risk posed by exposure to these pesticides. It is the level at or below which daily aggregate exposure to pesticides over a lifetime will not cause any significant risk to human health. It thus serves as a point of reference from which the potential health effects of these pesticides at other doses could be estimated. It is derived from the “no observable adverse effect levels” (NOAEL) of the chemicals and exposures at or below the RfD are considered acceptable by the USEPA [37] .
In this study, health risk estimates were calculated by integrating the pesticide analysis data from the study and the consumption rate of food (maize) in Ghana.
The following assumptions were adopted from the US Environmental Protection Agency’s guidelines:
a) Hypothetical body weight of 10 kg for children (0 - 1 yrs), 30 kg for children (1 - 11 yrs) and 70 kg for adults.
b) Maximum absorption rate of 100% and a bioavailability rate of 100% (USEPA, 1998).
c) Maize consumption rate in Ghana is 8.48 kg/day [38] .
Consumption of contaminants in maize was calculated based on its concentration in the food and on an estimate of the food consumption rates. Hence for each type of exposure, the lifetime exposure dose (mg/kg/day) was obtained by multiplying the residual pesticide concentration (mg/kg) in the food of interest by the food consumption rate in the country (kg/day) and dividing the product by the body weight (kg) [39] [40] . The hazard indices for children and adults were estimated as ratios between estimated pesticide exposure doses and the reference doses (RfD).
Pesticide residue data from maize samples (Table 3) were used for the health risk assessment. It is important to note that the data used represent the maximum concentrations of pesticide residues in the maize samples. Since a conservative approach is needed in dealing with risk assessment of multiple chemical compounds, it was more appropriate to consider the maximum levels of specific detected pesticides instead of their mean concentrations [40] . A hazard index HI < 1 is considered to have an unlikely adverse health effect, while HI > 1 is said to have probable adverse health effect on consumers.
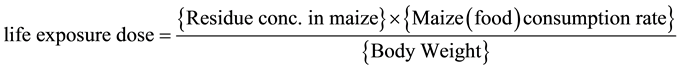

3. Results and Discussion
3.1. Field Survey
3.1.1 Demographic Characteristics
76 males representing 68.5% of respondents and 35 females representing 31.5% were interviewed (Table 1). The respondents in the survey included 77 farmers and 34 traders. Among these respondents, 91.8% have had some form of education while 8.2% have not had any form of education.
3.1.2. Respondents’ Usage of Lindane, Atrazine and Pesticides in General
All respondents (100%) used pesticides in the maize production process. However, only 18% used Lindane (a possible reason could be the ban status of Lindane being upheld) while 82% used Atrazine (Figure 2). The per-
![]()
Table 1. Demographic characteristics and level of education of respondents.
![]()
Figure 2. Respondents’ usage of lindane, atrazine and pesticides in general.
centage using Atrazine is as a result of Atrazine being a major herbicide used by most farmers interviewed in the study area.
These chemicals were easily found within the reach of the respondents. Majority got theirs from sales agents who themselves may not be well vexed in safe handling of these chemicals. Only 8.7% got them from extension officers while 19.7% got them from licensed agrochemical shops (Figure 3). Respondents also stored these synthetic chemicals in multipurpose storage structures together with food items and farm implements/tools. This practice is very unsafe as chemicals may contaminate food items and cause poisoning.
3.1.3. Practices, Toxicity Awareness and Symptoms among Respondents
72.1% of the respondents practice “cocktail”; which is a method of applying pesticides in mixtures. Tank mixtures of two or three were observed in this study. There were no specific instructions for farmers either from extension officers or on the label of these pesticides concerning tank mixtures. These farmers asserted that tank mixing was favourable because it saved time since they could apply more than one pesticide at a time. Moreover, they asserted that it also saved them cost and labour (Figure 4). “Cocktailing” is not a safe method of pesticide application because labelled instructions on pesticide containers do not cover mixtures of two or more pesticides and also give no information on the compatibility of active and inert ingredients. In a 2002 study for instance, it was observed that a mixture of fungicides, insecticides and water mineral content influenced the efficacy of individual pesticides against fungal pathogens and insect mortality respectively. Some tank mixtures were also found to induce phytotoxicity on tomato [41] . There is however limited information on the reaction and the effects of tank mixtures observed in my study. In another study, mixtures of insecticides generally resulted in the simultaneous development of resistant strains [42] . Farmers interviewed did not regard “cocktailing” as a less effective practice that could have adverse effects on their health and the environment; instead, “cocktailing” was believed to save time, labour cost as well as give high pest killing frequency. The observation of various tank mixtures is an indication of the lack of basic knowledge of safe pesticide application by respondents.
Moreover, 74.8% of respondents do not use protective clothing. Protective clothing such as goggles, gloves, long boots, overalls etc were not used by most of the respondents (Figure 4). Several reasons ranging from personal discomfort to lack of finances were given though a few wear long-sleeved clothes during the application process. One reason given by respondents for not wearing them is that the warm weather makes the wearing of protective gear impractical due to the possibility of heat stress. The results of a similar study in Egypt showed
![]()
Figure 3. Respondents’ sources and access to these chemicals.
![]()
Figure 4. Respondents’ practices and toxicity awareness.
that 100% of farmers did not use protective clothing. Reasons given were low level of knowledge about the safety measures, unavailability of protective devices at governmental agricultural associations and their high cost at private sector [43] . This finding is also consistent with the results of many other studies conducted in many parts of the world [42] [44] - [48] . Hot weather was among the causes of low use of protective clothing in a study conducted in the United States of America [49] .
In addition to the above, 52.3% of respondents also eat, drink or smoke during the pesticide application process (Figure 4). Most of the respondents regardless of their level of education practice this hazardous act. Similar behavior was reported in other developing countries [50] [51] . Though 47.7% do not practice this hazardous act, there is the need for education to reduce the numbers that practice this act. 91% of respondents do not have any form of training in pesticide mixing and application while 89.2% do not have access to advice on safe application practices from relevant authorities. The fact that majority get their chemical supplies from sales agents who themselves do not have any training in pesticide application may be a contributory factor. In addition to the above, 90.1% of respondents were unaware of the various diseases associated with exposure to these chemicals. In a similar study by [13] , it was reported that 85% of the respondents do not use protective clothing during pesticide application while 67% of them eat or drink when mixing or applying pesticides. It was also reported in the same study that 91% of the respondents had not received any training whatsoever on pesticides and the use of agrochemicals.
This study also revealed 80.2% (Figure 4) of respondents do not read instructions on pesticide containers. The main reason given was their inability to read and lack of awareness about any use these instructions could offer them. Farmers saw reading instructions as a time wasting venture. In a similar study in Egypt by Gaber and Abdel-Latif in 2012, 67% of applicators did not read instructions on containers. A study conducted in Ethiopia also found most pesticide applicators did not read instructions on pesticides packages due to illiteracy or they are just reluctant to read them [44] .
3.1.4. Symptoms of Pesticide Poisoning Experienced Respondents
Various symptoms were reported by respondents as seen in Figure 5. Headache was the most reported symptom followed by weakness and dizziness. Only 14% (16) of respondents reported no symptoms. In a similar study in Tanzania, [42] , reported 31% of respondents with dizziness, 31% with headache and 15% with stomachache. [52] also reported that farmers assume that pesticide poisoning symptoms are normal and as such they get used to them. In a similar study in Cote d’voire, Ajayi reported that applicators of pesticides tended to accept certain levels of illness as an expected and normal part of the work and thus do not report these symptoms at official health centers for treatment [53] . The symptoms recorded in my work however are not specific to pesticide exposure and could be due to other causes such as malaria and/or general fatigue.
3.1.5. Attitude and Awareness of Farmers towards Pesticides Hazards
From Table 2, respondents generally were less aware of the potential adverse impacts of pesticide deposition on the environment. Though 68.5% believe that pesticides application increases maize yield and over 74% believing that wrong use of pesticides could lead to human death, a significant amount (68.5%) were neutral about the notion that Pesticides contribute to elimination of beneficial organisms in soil and nature. These respondents are most likely unaware of the negative effects of these chemicals on biodiversity (bees, birds and soil ecosystem).
![]()
Table 2. Attitudes towards adverse impacts of pesticides.
Moreover, 68.5% were also neutral about the notion that Pesticide use could lead to Ground/Surface water pollution while 64.9% were neutral about the notion that Pesticides contribute to pollution and reduce soil fertility. However, 67.4% believe that the use of pesticides causes air Pollution by toxic compounds. This probably may be because they smell these chemicals during application as most do not use protective clothing. Though a study by Andersch and Anderson in 1991 showed that 31 different pesticides when applied at recommended doses had no long term influence on soil nitrogen mineralization, absence of national regulations on sustainable use might lead to detectable concentrations in ground and surface water [54] .
3.2. Levels and Distribution of Atrazine and Lindane (Organochlorines) in Maize
The overall mean recoveries from fortified samples were between 80% and 120%. The limit of detection (LOD) for Atrazine was 0.010 mg/kg while that of Lindane (Organochlorines) was 0.005 mg/kg. For quality control, a matrix blank was analysed with each extraction set. Recoveries for this study were all within the normal acceptable range of 65% - 120% [34] [35] [55] . A total of 9 composite samples representing 9 regions were analysed. Results are presented in Table 3 for Ashanti region (AR), Brong Ahafo region (BAR), Eastern region (ER), Northern region (NR), Central region (CR), Upper east region (UER), Upper west region (UWR), Volta region (VR) and Western region (WR). The maximum residue levels (MRLs) of these pesticides in maize for the European Union is also presented in Table 3.
The highest level of Atrazine (0.05 mg/kg) was recorded in the Ashanti region (AR) while Lindane and the other OCPs were below the detection limit of 0.005 mg/kg in this region. Atrazine in all the other 8 regional composite samples were below the limit of detection (0.010 mg/kg). Though Lindane was below detection limit for all samples, some amount of other organochlorines pesticides were detected in some samples. Endrin was found in central (0.006 mg/kg) and Brong Ahafo (0.007 mg/kg) regions while heptachlor was found in Brong Ahafo (0.027 mg/kg), Eastern (0.020 mg/kg), Central (0.021 mg/kg) and Upper West (0.023 mg/kg) regions. Alpha endosulfan (0.013 mg/kg) was also found in maize samples from the Eastern region. From the data (Table 3), concentrations of measured Atrazine in majority of the samples were below detection limit. The con-
![]()
Table 3. Levels of Lindane, Atrazine and other organochlorines in Maize samples from the study area (mg/kg).
centration measured for Ashanti region was also low (0.05 mg/kg). These relatively low levels of Atrazine may be due to soil characteristics as well as chemical and biological breakdown of Atrazine. Its persistence is also dependent on soil characteristics such as pH, organic matter content, moisture and texture [56] ; the relatively higher pH of Ashanti soils might be responsible for the measured levels of Atrazine found in maize samples in this region. Atrazine is a slightly basic herbicide and as such is easily absorbed in slightly acidic soils but persistent in high pH soils [57] [58] . At the relatively lowpH values in the other regions, chemical and biological breakdown occur faster; degrading Atrazine from the environment [59] .
Lindane below detection limit for all samples could be due to the ban status of this pesticide. Lindane use has been banned in Ghana since 2009 and its popularity and use has dwindled ever since. From
Figure 2 only 18% of respondents use Lindane and other organochlorines. Though Lindane was below detection limit, endrin (0.007 mg/kg for Brong Ahafo region; 0.006 mg/kg for central region), and heptachlor (0.027 mg/kg for Brong Ahafo; 0.020 mg/kg for Eastern region; 0.021 mg/kg for central region; 0.023 mg/kg for upper west region) exceed the MRL of 0.01 mg/kg set by the European Union for maize. Alpha-endosulfan (0.013 mg/kg) however is almost same as the EU-MRL for maize. Though the production and use of these chemicals (endrin, endosulfan and heptachlor) have been discontinued, their persistence in the environment as a result of their stability may be responsible for their presence in the samples from the Brong Ahafo, Eastern, Central and upper west regions. Heptachlor for instance was used from 1953 to 1974 as a soil and seed treatment to protect corn, small grains, and sorghum from pests [60] .
3.3. Health Risk Estimates
Table 3 represents atrazine, Lindane and other organochlorines pesticide residue data in maize from the study area while Table 4 represents the estimated dose values and health hazards associated with these chemical residues in maize from the study area. Hazard indices were computed for children between the ages of 0 - 1 years, 1 - 11 years and adults for three organochlorines (endrin, alpha-endosulfan and heptachlor for Brong Ahafo, Eastern, Central and upper west region) as well as Atrazine (for Ashanti region). Data analysis of health risk estimates indicated that all these chemicals do not pose a direct hazard to human health, although present in maize samples from the study area. Their potential for systemic toxicity in all age groups is thus low. The hazard in-dices for these chemicals (Table 4) also indicate an unlikely adverse health effect since the estimated doses exceeded the recommended reference doses for all the chemicals. These outcomes may be due to the fact that organochlorines pesticides Lindane, Endrin, Heptachlor, β-Endosulfan, Dieldrin, P’P-DDE, P’P-DDT, P’P-DDD, Δ-Chlordane, Methoxychlor, Delta-HCH, β-HCH, Endosulfansulfate, Alpha-Endosulfan and Aldrin have been banned from use in Ghana. However the little residues may be due to their persistence and stability in the environment. Atrazine levels in the Ashanti region may also be due to the fact that Atrazine is a slightly basic herbicide and as such is easily absorbed in slightly acidic soils but persistent in high pH soils [57] [58] . At the relatively low pH values in the other regions, chemical and biological breakdown occur faster; degrading Atrazine
![]()
Table 4. Estimated dose values and hazard indices of exposure to Atrazine, Lindane and other organochlorines residues in the study area.
from the environment [59] . In the Ashanti region however, the higher pH values may have led to the persistence of Atrazine in the environment hence its presence in small quantities in maize samples from this region.
Although residue levels of the organochlorines and Atrazine were below the maximum permissible intake of Codex Committee on pesticides residues [32] , as well as EU-MRLs [61] , there was no zero risk because pesticides were present in the maize samples though below detection limit in some cases. Since chemicals are generally persistent, volatile, lipophilic and bio-accumulative both in the environment and at each trophic level of the food chain, they have the potential to reach high concentrations through bio-magnification in the tissues of predators including humans, which are high on the food chain [62] . This can lead to adverse environmental and human health effects.
4. Conclusion
It is clearly seen from the results of the study that farmers and traders in the study area in Ghana do not follow appropriate safety precautions during pesticide application. Applicators thus experience clinicopathological conditions including nausea, vomiting, abdominal cramps, itching, stomach-ache, dizziness and headache. Though Lindane was below detection level in the study area, a detectable level of Atrazine was found in Ashanti region. Detectable levels of other organochlorines (endrin, heptachlor and alpha-endosulfan) were found in maize from Brong Ahafo, Eastern, Central and Upper west regions. The estimated dose for all the organochlorines and Atrazine do not pose a direct hazard to human health although present in the maize samples since their values were lower than toxic thresholds as well as reference doses. Residue levels of these pesticides are below the maximum permissible intake, but there was no zero risk because pesticides were present in maize samples though below detection limit in some regions. Thus due to the unsafe application practices by applicators, there exists a potential risk for systemic and carcinogenic health effects by these chemicals in the study area.
Acknowledgements
I am extremely grateful to the West Africa Agricultural Productivity Programme (WAAPP) of the Ministry of Food and Agriculture (MOFA) Ghana, for financing this project. Gratitude to the Ghana Standards Authority for availing their laboratory for sample analysis.