Copper Release Kinetics and Ageing of Insulation Paper in Oil-Immersed Transformers ()
1. Introduction
Excessive heat produced during high-load operation of oil-filled transformers leads to accelerated oxidation of oil and degradation of the cellulosic insulating material on copper windings. Increased acidity and humidity not only degrade the insulation capacity of the oil-paper system but also create a potentially corrosive environment within the transformer. This necessitates oil replacement or reconditioning at intervals. However, replacing the oil does not restore the paper to its original state, while it is generally accepted that the primary cause of the majority of ageing-related transformer failures is the degradation of the insulating paper and not of the oil. Therefore, by focusing either on the oil stability or on the paper stability alone, one gets only a one-sided view of the problem. A growing awareness of this initiated step towards studying the ageing chemistry for the oil-paper insulation system as a whole [1] -[4] . These studies suggest conclusively that various ageing processes occurring in oil-immersed transformers interact with each other, as shown in Figure 1.
For instance, the humid and hot environment in countries with a tropic climate may reduce the efficiency of cooling and facilitate accumulation of water inside the transformer, both due to more intense humidity intake and due to increased solubility of water in oil at elevated temperatures. This is especially true for free breathing transformers. Water is also a natural product of oil oxidation. Water and oxygen trigger corrosion of metallic parts. The subsequent release of transitional metal ions, in particular copper, will catalyse further oxidation of the oil [5] . Oil oxidation products, some of which have acidic character, will gradually accumulate in oil, contributing to protonic conductivity and eventually causing unwanted electrochemical processes, e.g. anodic oxidation of antioxidants, water and carboxylic acids to peroxides, etc. [6] . Increased acidity also spurs the degradation of paper, mainly by catalysing depolymerization and dehydration reactions. Paper degradation products, including polycarboxylic acids and furanic structures, are good complexing agents for transitional metal ions. The complexation may in some cases shift the electrochemical potential for copper dissolution to a level that makes acidic attack possible. This shows that, the ageing process is self-accelerating with time in a sense that the products generated at early stages of the process trigger other unwanted reactions later on.
2. The Chemistry of the Ageing Processes in Oil-Immersed Transformers
2.1. Oil Oxidation
Traditionally, the major emphasis in studies of transformer’s behaviour has been on the oil stability. It is commonly accepted that the oxidation proceeds via a free-radical mechanism involving the formation of peroxy radicals. Transition metal ions, notably copper, acting as initiators of the radical reactions, are known to increase the oxidation rate of hydrocarbons. The classical theory of the copper-catalysed oxidation process assumes the following initiation step,
Cu2+ + RH ® Cu+ + H+ + R・
R・ + O2 ® ROO・
followed by regeneration of Cu2+ ions by reaction with dissolved oxygen,
4Cu+ + O2 + 4H+ ® 4Cu2+ + 2H2O
This mechanism―homogeneous catalytic oxidation―is supported by the fact that the concentration of peroxides in oils oxidized by air increases with increasing the copper concentration [5] .
A concomitant increase in acidity promotes the release of new copper ions by leaching of thin oxide films invariably present at the surface of copper conductors. The typical thickness of such oxide films is 10 to 200 nm and it has a non-stoichiometric composition CuxO (x > 1). Apart from the two common copper oxides, Cu2O and CuO, corresponding to one- and two-valent copper, respectively, more exotic metastable compounds, such as
![]()
Figure 1. Interaction between different degradative processes occurring in oil-immersed transformers.
Cu64O, Cu8O, and Cu4O, were detected at initial oxidation stages [7] -[9] . It is doubtful, though, that the latter formulas describe individual chemical compounds―most likely, one deals with non-stoichiometric compounds formed by inclusion of oxygen into the crystal lattice of copper.
There are indications that Cu2O is more active in generation of radicals than CuO [10] . The two-valent copper ions, Cu(II), can be stabilized by complexation with a variety of bi- or poly-dentate ligands, such as aminoacids, hydroxycarboxylic acids, porphyrins, etc. The complexation will normally stabilize the two-valent state if the ligand has a free electron pair that can be accommodated by the vacant d-orbital of the Cu2+ ion (as is the case for amines, water, and carboxylic acids). However, in fresh insulating oils, especially in hydrotreated ones, there does not appear to be any such compounds, and therefore―at least in the beginning―the predominant state of dissolved copper is Cu(I). This conforms to the fact that the single-valent copper is stabilized by complexation with a variety of “soft” ligands whose electron orbitals are capable of hybridization with occupied d-orbitals of the Cu+ ion; a number of such complexes with olefins, carbonyls, acetylenes as ligands have been described in the literature [11] . The presence of single-valent Cu(I) ions in oxidized insulating oil has also been directly confirmed by neocuproine titration [10] .
In the excess of dissolved oxygen (40 to 50 ppm in open-breathing transformers), the Cu(II)/Cu(I) equilibrium is shifted towards copper (I) ions being oxidized to copper (II) ions triggering the radical oxidation process. However, if only one specific oxidation state is stabilized by complexation, the catalytic effect of copper may diminish, either because the resulting Cu(II) complex cannot abstract an electron from hydrocarbon, or because the resulting Cu(I) complex cannot be oxidized by oxygen.
In fact, some copper compounds, such as

or
(RNHCSNHR’)2Cu(OAc) (R,R’ = Bz, Ph, PhCHMe, p-MeOC6H4)
were found to inhibit oxidation by acting as scavengers of peroxy and alkyl radicals [12] [13] . On the contrary, copper chloride/crown ether complexes proved to be efficient oxidation catalysts, probably because crown ether protects both Cu(I) and Cu(II) from complexation with other ligands, making both valence states readily available for mediating electron transfer in red-ox processes.
In practice, oil oxidation can be effectively minimized by
1) use of antioxidants (to hinder the formation of radicals);
2) use of copper corrosion inhibitors (to hinder copper dissolution);
3) sealing and nitrogen-blanketing of transformers (to limit oxygen supply).
2.2. Copper Corrosion and Dissolution
Copper is the electrical conductor in many categories of electrical wiring. Transformers use copper winding wire. There exist several different types of chemical and electrochemical processes leading to copper dissolution:
1) Oxidative processes, e.g.
4Cu + O2 ® 2Cu2O
Cu + ROO・ ® Cu+ + ROO−
2) Reaction with acids in the presence of complexing agents or under oxidizing conditions,
Cu + H+ + L ® CuL+ + 1/2H2
2Cu + 2H+ + [O] ® 2Cu+ + H2O
where L denotes a chelating ligand, and [O] denotes an oxidants (it may be hydroperoxide, peroxyacid, oxygen, etc.)
3) Reactions with “corrosive sulphur” compounds, e.g.
2Cu + S ® Cu2S
2Cu + H2S + O2 ® Cu2S + H2O
2Cu + RSH ® Cu2S + RR + H2
2Cu + RSSR' ® Cu2S + RR + RR' + R'R'
The above heterogeneous processes rarely display any specific stoichiometry and may yield a variety of products due to non-selective radical recombination.
4) Galvanic corrosion:
Cu − e− ® Cu+
It should be noted that, based on the standard Red-Ox potentials, oxidation of water and many antioxidants present in oil is a thermodynamically preferential process. For instance, copper does not undergo electrochemical oxidation in aqueous solutions―it is rather water itself that is oxidized to H+ and oxygen. However, in insulating oils, the concentration of other oxidizable species near the “anode” surface may be depleted to such an extent that the electrochemical oxidation of copper becomes possible. Other possible anodic processes include oxidation of phenols, carboxylic acids, carbohydrates, and other oxidizable compounds naturally occurring in, or added to, the insulating oil [14] , e.g.
RCOO− − e− ® RCOO・ ® RR + CO2
Corresponding cathodic processes may involve reduction of dissolved oxygen to hydroxide ions, reduction of aromatics cycles to anion-radicals, and reduction of disulphides to mercaptide ions, the latter being a potentially dangerous process. Because of an extremely low ion concentration of such species in insulating oil, strong electrode polarization is result. Hence, under alternating current conditions, very low conversion degrees are expected. Let’s make some simple estimates. For instance, if the applied voltage is 1000 V, the electrode surface 10 cm2, and the oil resistance 10 GOhm, the resulting current density will be 1000 V/1010 Ohm per 10 cm2 = 10−8 A/cm2. Over a time of 30 years (which is 109 s), for instance, the equivalent Faraday ion flux will transfer 10C of electric charge per cm2, thereby removing or depositing 10C/96,485 C mol−1 » 10−4 mol of ions per cm2. This corresponds to etching away a surface layer of copper having a thickness of 7 μm only (10−4 mol∙cm−2 × 64 g∙mol−1/8.9 g∙cm−3 = 7 × 10−4 cm). The actual copper dissolution will be even less than that, because the Faraday current is going to be dominated by protons which have much higher mobility than copper ions.
The above estimates allow us to conclude that the fact that electrical stress speeds up copper release in transformers [15] is not likely related to electrochemical dissolution of copper as such but rather to anodic oxidation processes occurring at the surface of copper wire and leading to the formation of other aggressive species, such as oxygen and peroxides which may both attack copper chemically and trigger further chain reactions in the bulk. Besides that, the electrical stress in working transformers produces significant heat effects intensifying convective transport of reagents due to temperature gradients.
To minimize copper corrosion, a variety of metal passivators can be used. The most common in transformer oils are benzotriazole, mercaptobenzothiazole and their derivatives [16] -[18] . These compounds form dense and relatively impermeable surface films on the metal surface [19] -[21] . It doesn’t seem to be realized, however, that adding a metal passivator only creates a kinetic but not a thermodynamic barrier to corrosion: the corrosion is going to proceed at a lower rate but the end state―a corroded metal―remains unchanged.
2.3. Degradation of Paper
The heat-induced ageing of paper has been of concern to the paper industry for decades, and as a consequence, a large number of studies on its mechanism and factors influencing the ageing kinetics have been carried out, providing rather complete picture of the phenomenon [22] -[29] . Thus, it is well known that the thermal ageing of paper becomes especially rapid as the ambient temperature rises to 120˚C - 140˚C. For isolated bleached kraft pulps, the degree of ageing was reported to be nearly the same in nitrogen and oxygen atmospheres. However, for paper insulation in oil-filled transformers, the presence of oxygen has an accelerating effect on paper degradation [1] . The auto-oxidation of cellulose by atmospheric oxygen is believed to proceed through a free radical mechanism that generates peroxides and, subsequently, carboxylic acid groups [30] . Transition metal ions, specifically copper, have a pronounced catalytic effect on the oxidation.
Among the major factors having an adverse effect on the thermal stability of paper were mentioned humidity, acidity, and the presence of transitional metal ions, specifically copper and iron [4] [22] [26] [27] . Hydrolysis results in discoloration of paper and in a lowering of the degree of polymerization of the cellulose chain, and, consequently, a loss in paper strength [2] -[4] [23] .
One may hypothesize that at the conditions such as in power transformers: elevated temperatures and presence of radical species, cellulose in insulating paper undergoes a sequence of depolymerization, oxidation and dehydration reactions, e.g.

producing monosaccharides and certain unsaturated reactive intermediates, such as hexenuronic acid. Some quantities of hexenuronic acid are always present in kraft pulps used for the insulation as a result of the conversion of 4-O-methyl-D-glucoron acid side-groups of the xylan backbone under alkaline conditions used for the pulp production. As was shown previously [22] , hexenuronic acid groups are involved in further complex transformations yielding furanic structures in the end, e.g.
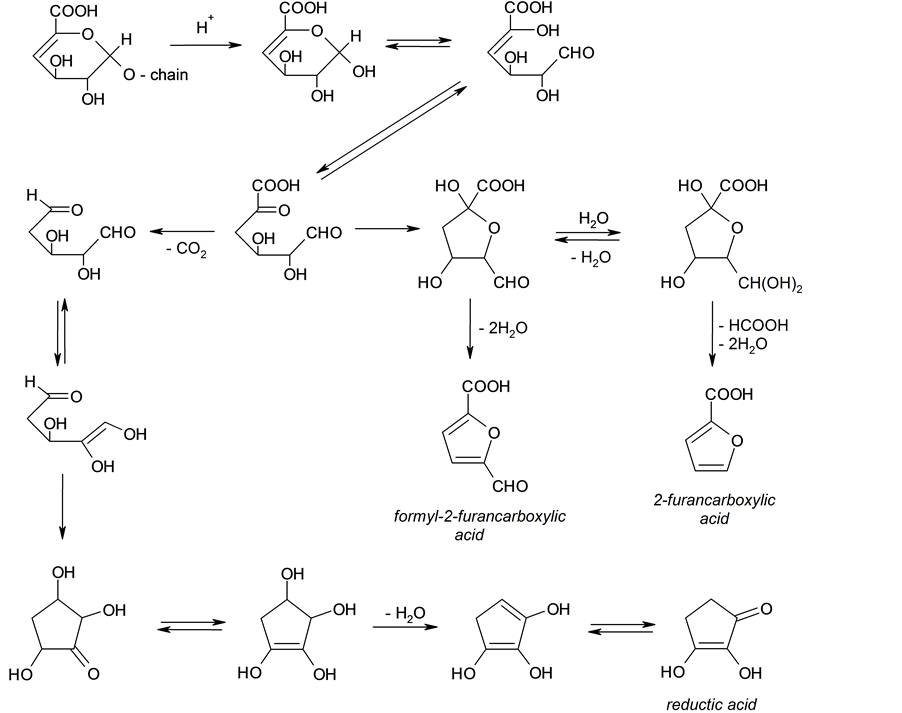
As a result, furanic structures are invariably present among carbohydrate degradation products in high-tem- perature processes such as wood pyrolysis, steam treatment, etc. [31] -[33] . The formation of furanic compounds, mainly furfural and hydroxy methyl furfural, is considered as an indication of the degradation of paper insulation in power transformers [24] [25] [28] [29] .
Deeper oxidation of cellulose yields a number carboxylic acids [27] (see Figure 2), many of which may act as complexing agents for copper.
![]()
Figure 2. Carboxylic acids produced as a result of oxidative degradation of paper [27] .
2.4. Water in Transformers
Water contamination of transformer oil is one the most common product quality deviations. The presence of water in oil compromises the insulating capacity of the oil/paper system, as can be demonstrated by a substantial drop in the break-through voltage and an increase in the dissipation factor of oil. Accumulation of water in transformer occurs mostly due to the ingress of atmospheric humidity and due to oxidation and dehydration reactions of oils and paper. Apart from degrading the insulation, water may also promote corrosion and bacterial attack. As the industrial experience shows, the problems become especially severe if the concentration of water in oil increases to such an extent that the phase separation occurs. In this case, water tends to condense inside the paper insulation or at the bottom of the transformer. Since the aqueous phase is polar, it selectively accumulates other polar substances, such as carboxylic acids and salts, creating ion-conductive bridges within the insulating material. This increases the risk of short-cuts causing a total transformer failure.
Understanding of the risks associated with the presence of water in transformers has led the development of technical solutions to avoid such problems. In most cases, adsorption, filtration or physical separation of water and other impurities, including sludge and colloidal matter, is attempted [34] -[38] .
3. Experimental
Two commercially available mineral insulating oils, an inhibitor-free transformer oil Renolin Eltec (FUCHS, density 0.868 g∙cm−3 at 20˚C; viscosity 10 cSt at 40˚C; total acid number < 0.01 mg KOH/g; pour point < −48˚C) and an inhibited transformer oil T-1500 (Bashneft, density 0.885 g∙cm−3 at 20˚C; viscosity 11 cSt at 40˚C; total acid number < 0.01 mg KOH/g; pour point < −45˚C) and paper wrapped rectangular copper wire (Cu-ETP, DIN 46434) for power transformers were used for the experiments on copper release kinetics.
Copper wire had three layers of insulating kraft paper and was cut into pieces of 10 cm length, so that all samples used in the ageing tests had the same dimensions: length 100 mm, width 6 mm, thickness 1.2 mm. The samples were placed in glass vial containing 50 ml of oil; two pieces per vial. Vials were thermostated at a desired temperature (150˚C). If needed, the vials were closed by ground-glass stoppers to minimize air intake; or air intake was controlled by bubbling air through the oil at a constant rate of 0.1 L/h. Aliquots of oil were sampled at intervals for testing.
In order to study the effect of oxidation products on copper release kinetics, 1 g of kraft paper impregnated by oil was aged for 2 weeks in an open vial placed in an oven at 150˚C. The resulting dark-brown product represents a crude mixture of cellulose and oil degradation products including furaldehyde, furancarboxylic acid, and a great number of other compounds. A small amount of the product (ca 0.5 mL) was added to 50 mL of fresh oil. Copper release from a bare copper wire was measured in a sealed-tube experiment at 150˚C and compared to the data obtained for pure oil under the same conditions.
4. Results and Discussion
4.1. Barrier Properties of Paper Insulation
The paper insulation present at the surface of copper wire creates a barrier to mass and heat transport processes. As can be seen in Figure 3, the copper release rate increases significantly if paper insulation is removed. In the sealed-tube experiment, inhibited and inhibitor-free oils post similar results. While using a thicker layer of paper certainly enhances its insulation capability, it also impairs the efficiency of heat removal. Hence, a compromise needs to be found.
4.2. Role of Early Oxidation Products in Copper Dissolution
As mentioned in the overview of the chemistry of insulation ageing processes, many oxidation products accumulating in oil as the ageing progresses are good complexing agents for copper ions. Therefore, it is logical to expect that copper dissolution rate increases with increasing “corrosiveness” of oil. This has been directly confirmed by comparing the copper release rates in fresh oil and in oxidized oil contaminated by a crude mixture of oil and paper degradation products, see Figure 4.
4.3. Effect of Additives on Copper Dissolution
So far, effects of common inhibitors on copper release kinetics have not been sufficiently studied.
Antioxidants are often used in oil formulations for improving the oxidation stability of the product [39] [40] . Dibutyl para-cresol (DBPC) is an effective radical scavenger, commonly used in inhibited oils. Another common additive is a copper corrosion inhibitor, such as 5-methyl-1,2,3-benzotriazole or tolutriazole (TTA). According to Maina et al. [41] and Amaro et al. [42] , some commercial products might have contained DBPC in
![]()
Figure 3. Barrier properties of the paper layer: the copper release kinetics from bare and paper-wrapped copper wire are compared. Sealed-tube experiment at 150˚C. The characteristic copper fluxes are ca 1 × 10−8 g∙m−2∙s−1 for the paper-wrapped wire and ca 1.0 × 10−9 g∙m−2∙s−1 for the bare wire.
![]()
Figure 4. Effect of paper degradation products on oil corrosiveness towards copper (sealed-tube experiment with bare copper wire, 150˚C).
combination with another undeclared additive, dibenzyldisulphide (DBDS). DBDS is an effective antioxidant of peroxide decomposer type, acting synergistically with DBPC and significantly improving the oxidation stability of the oil. Unfortunately, DBDS was found to be extremely copper-corrosive [41] -[44] , bringing about far more serious disruptions of transformer operation than oil oxidation can even do.
In sealed-tube experiments with restricted air ingress, the presence of DBPC has no effect on copper dissolution, while the presence of DBDS causes a significant increase in copper release rate, supporting the early reports that DBDS is corrosive towards copper.
In open-tube experiments under continuous air flow, the situation changes dramatically. First, much greater copper release rates are observed. Second, the presence of the phenolic antioxidant, DBPC, effectively hinders copper dissolution until the antioxidant reserve gets depleted by oxidation. Third, DBDS seems to become less corrosive in this case as it undergoes partial oxidation and the resulting sulfones are not copper-corrosive. In this connection, it should be pointed out that, while the presence of oxygen favours copper dissolution and higher acidity is normally associated with higher copper contents, it may not be the case when a sufficient amount of DBDS is present: even though oil is then better protected against oxidation, as reflected in a low total acid number of the aged oil, copper becomes vulnerable to corrosive attack by DBDS (see Figure 5).
4.4. Copper Release Kinetics
Copper dissolution in oil-filled transformers involves a number of reaction pathways. The fact that the dissolution rate increases under oxidizing conditions, e.g. in the presence of atmospheric oxygen, suggests that the metal oxidation plays an important role here. However, the dissolution rate is non-zero even in an inert atmosphere. This can be attributed to the dissolution of oxide films present at the surface of metal from the beginning. Based on ellipsometric data available in literature, the thickness of the oxide layer at the surface of copper is around 100 nm [45] . The surface area of the copper wire in our experiments was 2 × (0.006 + 0.001) × 0.01 = 2.8 × 10−3 m2, and hence, the surface layer contains ca 3 × 10−10 m3 of copper oxide.
Given the density of this substance of ca 6.4 × 103 kg/m3, the mass of the oxide film should be around 2 mg. The complete dissolution of such a film would produce as much as 20 ppm of copper in our experiments, which is one to three orders of magnitude greater than the actual concentrations measured (in the ppb range). This proves that, during the experimental time, the initial oxide film has not yet been dissolved completely. What is often considered as the reaction of metal copper in reality appears to be the reaction of copper oxide.
The question arises why the presence of oxygen accelerates the “copper” dissolution once the metallic surface remains buried under the oxide film. The explanation is rather simple: First, the oxide film is not absolutely impermeable―copper oxide does not form passivating films as does, for example, alumina. Second, more importantly, the concentration of dissolved oxygen is directly proportional to the partial pressure of oxygen in the
![]()
Figure 5. Effect of DBDS on copper release and oil oxidation kinetics. (Open-tube experiments with a DBPC-inhibited commercial product T-1500 and paper-wrapped copper wire at 150˚C, the concentration of DBDS was 500 ppm).
surrounding atmosphere (air or a nitrogen blanket). In free-breathing transformers, it may reach 40 - 50 ppm; and in sealed transformers it still may be a few ppm. Since the rate of oil oxidation increases with increasing the oxygen concentration, more oxidation products capable of etching the oxide film is formed per unit time, and hence, the copper release rate also increases.
Let’s formulate the corresponding kinetic equations for several feasible kinetic scenarios.
Scenario 1: Let the dissolved copper be generated by dissolution of the oxide film. In this case, the flux of dissolved copper at the x = 0 is given by
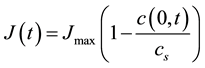 (1)
(1)
where Jmax is the maximum flux achieved under no diffusional restrictions, cs is the saturation concentration of copper oxide in hydrocarbon media, and c(0, t) is the actual concentration of copper near the surface (see Figure 6). The concentration profile of copper within the insulation paper, i.e. in the range 0 < x < L, is given by the diffusion equation,
 (2)
(2)
with the boundary conditions
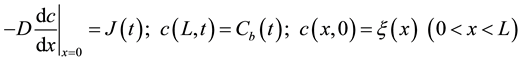 (3)
(3)
where D is the diffusion coefficient of copper in paper, 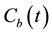 is the concentration of copper in the oil phase and
is the concentration of copper in the oil phase and 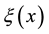 is the initial concentration profile of copper within the paper layer at the beginning of the experiment. Adding the mass conservation requirement,
is the initial concentration profile of copper within the paper layer at the beginning of the experiment. Adding the mass conservation requirement,
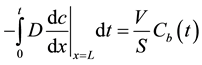 (4)
(4)
where V is the volume of oil and S is the surface area of the copper wire, one can calculate the copper release kinetics.
![]()
Figure 6. Mass transport processes occurring in the paper-oil insulation system during the ageing process.
If dissolution goes rapidly, so that the saturation concentration is constantly maintained near the wire surface (i.e. c(0, t) = cs), the diffusion profile can be approximated by that for diffusion from a distributed source. The saturation is achieved over a time of D(cs/Jmax)2 and is localized within a layer of thickness Dcs/Jmax. Afterwards,
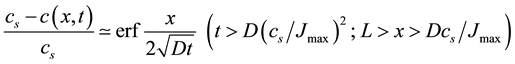 (5)
(5)
i.e. one has a descending diffusional profile with the concentration of copper declining within the subsequent paper layers.
If the diffusion coefficient is, for example, 10−10 m2/s and the thickness of the paper insulation 1 mm, it would take just a few hours for diffusion front to advance to the top paper layer and copper to start to be released into the adjacent oil phase.
Further, if the distribution of copper within the insulation layer separating two adjacent copper wires is concerned, a U-shaped concentration profile is to be expected,
![]() (6)
(6)
and has been observed experimentally elsewhere [46] .
If, on the contrary, dissolution goes slowly, so that there is only a little change in concentration of dissolved copper over a time of L2/D, a nearly linear concentration profile will be maintained,
![]() (7)
(7)
for a copper wire in contact with the oil phase, and a uniform copper concentration profile will be maintained in the insulation between the adjacent copper wires,
![]() (8)
(8)
If the binding of copper to the surface of paper is taken into account, the form of the concentration profile will not change, but the effective diffusion coefficient will decrease to D/(1 + K), where K is the corresponding adsorption constant.
Scenario 2: The copper release is mediated by a corrosive substance present in the oil phase from the beginning. Examples of such substances are “corrosive sulphur compounds”, e.g. DBDS.
From a mathematical viewpoint, this is a more complex situation since multiple fluxes are involved. The Maxwell-Stefan formalism is an appropriate tool for describing the mass transport in this case. Let cc and Nc denote the molar concentration and the molar flux of the corrosive substance diffusing towards the metal surface, and cp and Np denote the same for the corrosion product diffusing in the opposite direction, towards the oil phase. In this case, the governing equations are as follows,
![]() (9)
(9)
Notice that there are two sorts of the diffusion coefficients: Dij describe the cross-interaction between the fluxes of the corrosive substance and the corrosion products, and![]() , referred to as the Knudsen diffusion coefficient, describes the interaction with the medium (insulating paper) through which the diffusion takes place.
, referred to as the Knudsen diffusion coefficient, describes the interaction with the medium (insulating paper) through which the diffusion takes place.
The above equations must be complemented by a kinetic equation describing the generation of the corrosion product at the surface. In general, one may distinguish:
1) diffusion-controlled kinetics;
2) activation-controlled kinetics;
3) mixed kinetics.
Let’s briefly analyse all the three cases:
1) In the diffusion-controlled case, all the corrosive substance that penetrates to the metal surface is immediately converted into the corrosion product, which migrates back into the oil phase. As shown before, the characteristic diffusion time through a 1 mm thick layer of insulating paper does not exceed a few hours. It is during this initial interval of time that the copper release will reveal the classical diffusion kinetics with the amount released being proportional to square-root of time. Afterwards, the corrosive substance is going to be consumed at a constant rate of Dcc/L. Accordingly, the amount of copper released increases linearly with time―this may be mistakenly considered as a sign of an activation-controlled process. Indeed, by hindering the reagent transport, the paper layer acts as a kinetic barrier to copper release.
If, for instance, D = 10−10 m2∙s−1; L = 1 mm and cc = 1 mol∙m−3 (typical at treat levels of around 100 ppm), the consumption rate will be around 10−7 mol∙m−2∙s−1. The corresponding copper release will be then of the order of 10−5 g∙m−2∙s−1. In our experiments, the exposed surface area was 3.2 × 10−3 m2, the oil volume 50 mL. Hence, in the diffusion-controlled regime―provided that the corrosive substance and the corrosion product have comparable diffusivities―the expected copper release rate should be around 500 ppb per day, 50 times exceeding the characteristic values measured experimentally (ca 10 ppb per day). The lower-than-expected release rate may be attributed to 1) the binding of the corrosion product by the insulating paper, as this causes a drop in diffusivity; and to 2) the existence of an activation barrier for the conversion of the corrosive substance into the corrosion product.
To figure out the actual cause, experiments were carried out with a copper wire stripped of the insulation paper and DBDS as a copper-corrosive substance. For bare copper wire, the copper release rate was almost one order of magnitude greater than for paper-covered wire, thus suggesting that the corrosion product―copper sulphide in the case in hand―was retained by paper.
2) In the activation-controlled case, the reaction rate at which the corrosive substance is converted into the corrosion product depends on the local concentration of the reagents and reaction products in the conversion zone. For instance, if diffusional limitations are eliminated by agitation and the corrosion product does not form a passive surface film, the conversion rate often follows the simple first-order kinetic equation,
![]() (9)
(9)
If a passive surface film is formed, the second-order kinetic is more common,
![]() (10)
(10)
where ![]() indicates the saturation concentration of the corrosion product. Usually,
indicates the saturation concentration of the corrosion product. Usually, ![]() , where VM is the molar volume of the corrosion product. Once the saturation concentration is reached, the conversion rate virtually goes down to zero. Examples of such reactions are the reaction of steel with concentrated sulphuric acid, in which case a passive film of iron sulphate protect steel from acid attack, and the reaction of aluminium with oxygen, in which case a surface film of alumina protects the metal from oxygen attack. DBDS, however, does not form a protective film on copper and the corrosion will proceed until complete conversion of DBDS into copper sulphide. Surface films produced by metal passivators of TTA type appears to be semi-permeable, as significant metal release are observed.
, where VM is the molar volume of the corrosion product. Once the saturation concentration is reached, the conversion rate virtually goes down to zero. Examples of such reactions are the reaction of steel with concentrated sulphuric acid, in which case a passive film of iron sulphate protect steel from acid attack, and the reaction of aluminium with oxygen, in which case a surface film of alumina protects the metal from oxygen attack. DBDS, however, does not form a protective film on copper and the corrosion will proceed until complete conversion of DBDS into copper sulphide. Surface films produced by metal passivators of TTA type appears to be semi-permeable, as significant metal release are observed.
3) In the mixed kinetic regime, which is most common in practice, both diffusional and activation limitations play a role. The corrosive substance diffusing to the metal surface is only partially converted into the corrosion product. In this case, the Stefan-Maxwell mass transport Equations (9) should be coupled with appropriate boundary conditions describing the surface reaction kinetics, e.g.
![]() (11)
(11)
where qi (i = c, p) are the degrees of surface filling by the adsorbed corrosive substance (subscript c) and the corrosion product (subscript p), and ![]() and
and ![]() (i = c, p) are the adsorption and desorption rate constants for the corrosive substance and for the corrosion product, respectively;
(i = c, p) are the adsorption and desorption rate constants for the corrosive substance and for the corrosion product, respectively; ![]() is the conversion rate constant; and lc, lp and lcp are empirical parameters, taking into account the energy of lateral interactions between the corresponding adsorbed species.
is the conversion rate constant; and lc, lp and lcp are empirical parameters, taking into account the energy of lateral interactions between the corresponding adsorbed species.
The above kinetic equations should be complemented by the mass conservation constraint
![]() (12)
(12)
which demands that the change in the total amount of substance adsorbed to the surface be equal to the difference between the incoming and outgoing fluxes.
The examples of reactions following the abovementioned kinetics equations are those of etching the surface oxide films by carboxylic acids, sulfonic acids, benzotriazoles, and other surface-active organic compounds capable of forming complexes with copper, e.g.
2RSO3H + Cu2O ® 2RSO3Cu + H2O
Scenario 3: The copper dissolution occurs primarily due to reaction with a corrosive substance which is produced in oil in the course of ageing. Examples of such corrosive substances are carboxylic, hyrdoxycarboxylic and β-keto acids originating either from paper or from oil, as well as hydroperoxides originating from oil oxidation. These substances, in combination, can etch both copper oxide and metallic copper according to the reactions,
Cu2O + 2H+ ® 2Cu+ + H2O
2Cu + ROOH + 2H+ ® 2Cu+ + ROH + H2O
The oxidation rate at a given temperature is known to increase with increasing the partial pressure of oxygen. Under the normal conditions, mineral oil dissolves 40 to 50 ppm of oxygen. If one runs a sealed-tube experiment and the dissolved oxygen is entirely converted into carboxylic groups, the resulting acid concentration will be 1 - 2 mmol∙dm−3, corresponding to a total acid number around 0.06 - 0.12 mgKOH/g. In practice, the total acid number of oils aged in a sealed-tube experiment is always less than the above estimate because of the formation of oxidation products other than carboxylic acids.
In a sealed-tube experiment, the concentration of a corrosive substance produced as a result of oil oxidation will increase with time according to the equation,
![]() (13)
(13)
where k is the oxidation rate constant and ![]() is the maximum amount of the corrosive substance a given amount of oxygen can produce.
is the maximum amount of the corrosive substance a given amount of oxygen can produce.
In an open-tube experiment, when the concentration of dissolved oxygen is maintained constant by e.g. bubbling air through oil, the concentration of a corrosive substance produced as a result of oil oxidation will increase with time according to the equation,
![]() (14)
(14)
where ![]() is the volume fraction of oxygen in air.
is the volume fraction of oxygen in air.
If copper dissolution were caused by a corrosive substance generated by oil oxidation, the copper release rate would have increased with time, producing a parabolic rather than a linear release vs time kinetic curves. This does not appear to be the case. Even though copper release rates measured under oxidizing conditions (air) are found to be consistently higher that those measured under neutral conditions (nitrogen), the concentration of dissolved copper increases approximately linearly with time in both cases. Therefore, there must exist another kinetic step―e.g. desorption of reacted copper―that limits the copper release rate.
The typical kinetic curves corresponding to above-mentioned kinetic scenarios are compared in Figure 7.
It should be noted that the dissolved copper tends to concentrate in sludge, the amount of which increases in the course of oxidation. This explains why the copper content in oil may eventually pass through a maximum and then start to decline―this reflects the complex interplay of the copper dissolution and the sludge precipitation processes.
4.5. Estimation of the “Activation Energy” for the Copper Release Process
In fact, it is inappropriate to talk about the activation energy of a multi-pathway process: as has been pointed out in the previous sections, there are a number of reaction pathways leading to copper accumulation in oil. Let, for instance, there be two parallel reactions, having the activation energies E1 and E2, respectively. Then, the relative yields of those reactions change with temperature as![]() . In other words, the apparent “activation energy” of the entire process changes with temperature, making the simple Arrhenius law inapplicable. Since, in the present study, no attempts have been made to delineate individual contributions of various ageing reactions, only general observations regarding the changes in the copper release kinetics with temperature are reported:
. In other words, the apparent “activation energy” of the entire process changes with temperature, making the simple Arrhenius law inapplicable. Since, in the present study, no attempts have been made to delineate individual contributions of various ageing reactions, only general observations regarding the changes in the copper release kinetics with temperature are reported:
1) Oxidative versus non-oxidative cases. In sealed-tube experiments, the copper flux increases by about 10 times on increasing the temperature from 20˚C to 120˚C. In open-tube experiments, the equivalent flux increase is one order of magnitude greater. This suggests that, under the oxidative conditions, the copper release is significantly influenced by another chemical process with a higher activation energy. This empirical finding well conforms to the fact that the activation energy for the hydrocarbon oxidation initiation reaction (ca 40 kcal∙mol−1 according to [40] ) is much greater than the activation energy for typical complexation and diffusion processes (usually, 1 to 10 kcal∙mol−1, see e.g. [47] [48] ).
2) Wrapped wire versus bare wire. The removal of paper from the copper wire somewhat reduces the “activation energy” of the copper release process. This is what is expected for a sequential process including of two or more activated stages: Indeed, the removal of paper eliminates the diffusion barrier and precludes the adsorption of copper onto the paper surface.
5. Conclusions
1) The ageing of the oil-paper insulation system in oil-immersed electrical current transformers involves several inter-related reaction pathways, the most important of which are a) thermal ageing of the insulating paper;
![]()
Figure 7. Characteristic copper release curves for various kinetic scenarios.
b) oxidation of the oil; and c) corrosion of copper winding followed by accumulation of corrosion products within the insulating paper layer.
2) The relative importance of those processes varies greatly depending on the operational conditions, the base oil quality, the additive package, and the transformer design. For instance, in highly loaded or overloaded transformers, operated at a high temperature, the thermal degradation of the insulating paper is unavoidable, no matter which oil and additives are used. In normally loaded open-breathing transformer, oil oxidation will occur. Acidic oil oxidation products accumulating in oil not only affect the dissipation factor and insulating capability of the oil itself but also promote depolymerization of cellulose and etch metal. In this case, using inhibited oils containing antioxidants remedies the problem: inhibited oils are found to perform much better than non-inhib- ited ones in the majority of tests. However, it should be kept in mind that additivation is sometimes done for a mere purpose of passing certain unified quality standards―often lacking foresight of actual application scenarios. As a result of that, an additive which proved to be highly efficient in open breathing transformers may not have the same effect when used in sealed-type transformers. Ideally, additivation strategies should match end- use scenarios.
3) Copper release kinetics are strongly influenced by oil quality, additives and ageing conditions. Unsaturated hydrocarbons, oil oxidation products and paper degradation products all play a role in copper transportation. The mixed diffusion-activation controlled kinetic mechanism is applicable in most cases. Use of appropriate antioxidants, preferably in combination with a metal inhibitor, allows one to effectively minimize copper dissolution, specifically in open-vial experiments and in corrosive environment. The presence of water in oil slightly accelerates copper release, due probably to faster cellulose degradation in humid environment.
NOTES
*Corresponding author.