Mineralogical and Geochemical Characteristics of Caprock Formations Used for Storage and Sequestration of Carbon Dioxide ()
1. Introduction
The development of carbon capture and storage (CCS) technique aims to reduce the atmospheric concentration of greenhouse gases emitted by industrial activities [1] . The carbon dioxide (CO2) captured from large stationary sources can be safely injected and stored in appropriate geological formations, such as deep saline formations and depleted oil and gas reservoirs [2] . These geological formations are considered as the most stable in the CO2 storage process on long term scale [3] . Four trapping and storing mechanisms are widely discussed in the CCS literature: residual, structural, mineral trapping and hydrodynamic [4] . After CO2 injection, the cover rocks constitute the first barrier preventing the migration of CO2 outer the geological reservoir. Generally, the most effective caprocks are siliciclastics (clay), evaporites (gypsum, anhydrites, halites) and organic-rich rocks [5] . The long-term confinement of CO2 injected in the deep reservoir will be crucially dependent on cap rock and CO2 interaction. The reaction between CO2 and two-caprock samples of carbonate and clay-types has been studied in a laboratory reactor under the conditions of geological storage [6] . It has been shown a change in mineralogical compositions for the two samples. Using gravimetric method, the sorption capacity and kinetics of CO2 have been measured among the clay minerals (montmorillonite, illite, and sepiolite) [7] . A thermodynamic study of CO2 adsorption has been performed on different adsorbents (Clay, Jurassic evaporates and Triassic sandstone) [8] . This study evaluated the best material able to absorb the maximum of CO2 and therefore to optimize the choice of the storage site. The CO2-brine-rock interaction can also generate some new mineral precipitation so as to change the properties of the reservoir. The properties change can influence the physical and chemical retention mechanisms of CO2 (drainage and imbibitions) [9] . Pressure and temperature effects on the reactivity of the host rock minerals with supercritical CO2 have been studied by Regnault et al. [10] . The authors have discussed CO2 storage capacity, mechanical reservoir behavior and chemical alteration. Other experimental studies and theoretical methods have been interested in the forsterite dissolution and magnesite precipitation at geological storage conditions [11] . Their experiments offer insights into the effects of relevant temperature and CO2 pressure levels on mineral dissolution and carbonate precipitation. The chemical modification of the solid phase has been observed by scanning electron microscopy (SEM), infrared spectroscopy (IR), and X-ray diffraction techniques.
The clay cover rock was used to determine the change in electrical and capillary forces between clay, CO2 and water [12] . This change leads to chemo-hydro-mechanical phenomena that could facilitate CO2 break and advection through porosity cap rocks. Computational models [13] offer a means of comparing and selecting storage reservoirs (storage capacity, escape potential, risk analysis escape routes and storage). These models require an understanding of minerals clay effects on scales variety. Therefore, it is crucial to understand the cover rocks nature in order to assess the reactivity of these minerals with respect to CO2.
The main objective of the present experimental study is to examine the characteristics of some geological cap rocks from real site located in southern region of Tunisia. It aims to identify common features that may impact long-term CO2 storage. Four different simples of clay-type will be chosen for the experiments. Different techniques will be used to characterize the physical and chemical properties at different observation scales.
2. Materials and Methods
The present study deals with chemico-mineralogical characterization and technological properties of clay minerals, raw material collected from real site located in the city of Gabes in southern Tunisia. The site from which the samples are taken is drawn in Figure 1 [14] .
X-ray diffraction and infrared spectra allow us to describe the mineralogical compositions of the simples. The samples structure will be investigated using scanning electron microscope. While measurement of the mass change will be delineated by thermal analysis. However, the fluorescence measurement of the samples will be achieved by photoluminescence.
X-ray diffraction analysis was carried out by a “Philips MPD1880-PW1710” diffractometer using 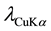 radiation, in the 2˚ - 80˚ interval with a step size of 0.02˚ and counting time of 20 s/step. The quantification phase was performed on one sample by the Rietveld method (R-QPA), using a PANalytical X’Pert High-Score Plus program. The chemical analyses and composition of the rock samples and clay minerals were examined using a JEOL JSM 5600LV scanning electron microscope (SEM) coupled with an energy dispersive spectrometer (EDS) (Bruker AXS Microanalysis). Infrared spectra were obtained using a Vertex 70-RAM II Bruker spectrometer (Bruker Analytical, Madison, WI). Differential and thermo gravimetric analyses were obtained using
radiation, in the 2˚ - 80˚ interval with a step size of 0.02˚ and counting time of 20 s/step. The quantification phase was performed on one sample by the Rietveld method (R-QPA), using a PANalytical X’Pert High-Score Plus program. The chemical analyses and composition of the rock samples and clay minerals were examined using a JEOL JSM 5600LV scanning electron microscope (SEM) coupled with an energy dispersive spectrometer (EDS) (Bruker AXS Microanalysis). Infrared spectra were obtained using a Vertex 70-RAM II Bruker spectrometer (Bruker Analytical, Madison, WI). Differential and thermo gravimetric analyses were obtained using
![]()
Figure 1. Location of El Hamma region in the city of Gabes located in southern Tunisia from which samples of clay- type are collected.
an (ATG-DSC) STA 449C Netzsch instrument operating in helium atmosphere and heated at a rate of 20˚C from room temperature to 1500˚C. Photoluminescence (PL) measurements were collected on a Jobin-Yvon Fluorolog 3 spectrometer using a Xenon lamp (500 W) at room temperature.
3. Results and Discussion
3.1. X-Ray Diffraction (XRD)
The mineralogical profile of the clay sample can be examined using X-ray diffraction in order to identify the crystalline components present in the clays. The XRD patterns of the clay samples had similar mineral compositions, consisting mainly of illite, calcite and quartz.
Figure 2 shows the X-ray diffraction pattern of the clay samples. The following mineralogical phases were identified: calcite (3.85 Å, 3.03 Å, 3.55 Å), illite (9.79 Å and 9.79 Å) and quartz (4.26 Å and 3.35 Å) as the principal minerals. Other secondary mineral phases are also found in this clay such as dolomite (2.88 Å). The mineralogical compositions of raw materials obtained with XRD analysis summarized in Table 1, indicate that the mineral association is the same in all cases and corresponds to the mixture of Calcite, Illite, Quartz, Dolomite and Albite.
3.2. Scanning Electron Microscope (SEM)
The surface topographies of different studied compounds are analyzed by scanning electron microscope (SEM) and energy dispersive X-ray spectroscopy (EDS) (Figure 3). The SEM imaging shows that clay occurs as crystals of variable sizes of undefined outlines and edges. Then, the particle morphology is shown to be laminated. EDS analysis allows us to identify that the samples of clay-type are dominated by Si, Cl, Na, and O.
![]()
Table 1. XRD mineralogy analysis (wt%) of different samples of clay-type.
3.3. Infrared Spectroscopy
Infrared technique has been frequently used for the identification of natural clay minerals, the minerals such as kaolinite, illite and quartz were identified by comparing the observed wave numbers with available literature [15] and [16] . The absorption profiles of the four chosen clay samples, S1-S4, are roughly similar, as depicted in Figure 4, showing the presence of OH-stretching bands in the vicinity of 3400 cm−1. The Si-O stretching bands near 1000 cm−1 indicate the presence of illite [17] . The characteristic band at 1428 cm−1 suggesting the presence of carbonate (calcite or dolomite) [15] . The appearance of intensity at 794 and 779 cm−1 in all spectra is considered an indication of quartz [16] . The bands at 669 cm−1 and 647 cm−1 confirmed the presence of plagioclase (albite or anorthite). However, the band at 1625 cm−1 is attributed to hydrogen bonded water and corresponds to the position of the water bending mode of liquid water [18] . Indeed, the stretching vibration of OH bonds at 3630 cm−1 clearly indicate the presence of kaolinite [19] .
![]()
Figure 4. Infrared spectra of different samples.
3.4. Thermal Analysis
In accordance with related published papers [20] and [21] , our results obtained with differential thermal analysis (DTA) and thermal gravimetric analysis (TGA) for simples of clay-types are illustrated in Figure 5. For all the selected samples, DTA curves reveal similarities in low-temperature range. Moreover, one can observe an endothermic peak system at low temperatures (<200˚C) corresponding to the loss of hydration water. A strong endothermic peak appears at the temperature range of 509˚C - 515˚C which is related to the departure of constitution water resulting from the dehydroxylation of clay minerals [21] . Another endothermic peak is observed at about 730˚C due to the decomposition of carbonates [22] . The mass loss associated to this peak is summarized in Table 2.
3.5. Fluorescence Spectra
Regarding preliminary experiments performed prior to CO2 storage, Figure 6 provides cartographies photoluminescence PL (excitation-emission) in false colors performed on the four chosen simples. For more clarity, cartography colors going from blue to red represent the increasing of the PL intensity depicting steady-state PL emission versus PL excitation (PLE). The emission patterns were varied among samples allowing their classification. The response of the four samples is situated in the spectral region from 330 to 480 nm for an excitation wavelength range 220 - 280 nm. These cartographies show also that the intensity of emission is maximized at the spectral region varying from 440 to 470 nm (red color) for an excitation wavelength between 240 and 260 nm. The prompt view of these maps shows qualitatively that the PL spectrum is broad in the case of S4 compared to the other samples (S1, S2 and S3).
4. Concluding Remarks
The present experimental research aimed to examine the chemical characteristics of cap rock formations considered for CO2 storage process. Different characterization techniques have been used to characterize the cover rock. Experimental results obtained with DRX demonstrated the presence of quartz, illite, Calcite, and Dolomite for the different selected samples of clay-type. The presence of these minerals was also confirmed by IR analysis.
![]()
Table 2. The mass loss associated to the endothermic peak for different samples of clay-type at different temperature levels.
EDX analyses justified that these clays were rich in Si, Cl, Na and O accompanied by a significant number of iron oxides. The DTA curves of clay samples revealed that three endothermic peaks were mainly due to the loss of H2O from clay minerals and from the carbonates decomposition. Florescence results indicated that the spectrum was broad in the case of sample S4. Moreover, the obtained experimental results offered us a means of evaluating, comparing, and selecting storage reservoirs on criteria such as ease of injection, storage capacity, migration, and escape of CO2 from a potential reservoir.
Acknowledgements
The authors would like to express their gratitude to the members of the Physics Complex Systems Laboratory at the University Picardy Jule Verne in France for their kind help in the characterization process of selected samples with fluorescence spectra and thermal analysis.
One of the authors (Mr. Hedi Jedli) is grateful to the Tunisian Ministry of Higher Education and Scientific Research for the grant in the framework of “Bourse d’alternance”.
NOTES
*Corresponding author.