Recycling of Cobalt by Liquid Leaching from Waste 18650-Type Lithium-Ion Batteries ()
1. Introduction
Rechargeable lithium-ion batteries as a new generation of green, non-pollution chemical energy storage device are widely used in portable electronic apparatus and vehicles. However, waste LIBs contain heavy metals, organic chemicals and plastics, which will bring environmental pollution. Therefore, the recycling of major components from spent LIBs is considered to be a beneficial way to prevent environmental pollution and as alternative resource of cobalt [1] . 18650-type power batteries are widely used in small portable devices, increasing along with the rise of electric vehicles in Tesla [2] . The spent lithium ion batteries contain 5 - 15 wt.% cobalt and 2 - 7 wt.% lithium as important constituents, which are an active cathodic material. The waste 18650 type lithium ion batteries contain precious metal elements cobalt with a certain toxicity as important constituents, which are an active cathodic material. Since cobalt is a relatively expensive material compared to the other battery constituents and can be widely used in new LiBs electrode materials, its recovery is one of the primary objectives in the recycling of spent batteries [3] [4] . Figure 1 is a 18650 type battery price polyvalent metallic element [5] . It is not difficult to see from Figure 1 that the price of the cobalt is more expensive than the other battery constituents. Therefore the recovery and utilization of cobalt have significant economic and social environmental benefits [6] .
Separation and Recovery Technology 18650 cobalt lithium ion battery mainly includes three steps: 1) the waste batteries discharge, stripped shell, simple crushing, after screening to obtain an electrode material, or simply crushed after firing to remove organic matter to obtain an electrode material [7] ; 2) the material obtained in the first step of the electrode is dissolved in the leaching of various metals into solution wherein both the cobalt and nickel represent as trivalent forms. Leaching sub-step dissolution method and two-dissolution method: direct step by acid leaching dissolution method, all the metal was dissolved in acid, and then isolated using a number of different purification and recovery; two-step method is to use an alkali leaching aluminum and recovered, and then use acid leaching surplus metal oxide, followed by the similar treatment as the first step [8] ; 3) the solution (leachate) dissolved metal elements in the solution is separated for recycling or direct synthesis anode material. Separation and recovery methods are chemical precipitation, salting out, ion exchange, extraction, electrochemical method, respectively cobalt or lithium-containing compound [9] . E.M.S. Barbieri et al. [10] have investigated recycling cobalt from the cathodes of spent Li-ion batteries as β-Co(OH)2, obtaining Co3O4. β-Co(OH)2 with a hexagonal structure by using chemical precipitation or electrochemical precipitation. This method does not directly recovered cobalt. In this paper, we recovered directly cobalt by electrodeposition.
2. Experimental
Experimental material: Waste 18650 lithium-ion battery, industries concentrated sulfuric acid, industrial grade sodium borate. Hand split waste 18650 lithium-ion batteries, remove the interior material with pliers, and finally the positive and negative material separated and dried 24 h at 60˚C. The obtained positive electrode material with 10 mol/L sulfuric acid leaching 1h, and filtered to give leachate. Spectrophotometric determination of Co2+ concentration in the solution (722S spectrophotometer, Shanghai Precision Scientific Instrument Co., Ltd.) with cobalt ions spectral qualities, using X-ray diffraction (Japan Rigaku company DM/ax-IIIA) salting crop products phase analysis. Process is shown in Figure 2, ICP analysis content of the test solution of various metal elements.
3. Results and Discussion
3.1. Effect of pH on the Leaching Process
When the solution of sulfuric acid concentration is too low, even at boiling temperature, leaching reaction is also extremely slow. Therefore, the leaching temperature was set at 70˚C, under the conditions of the reaction time of 1 h, acidity leaching test, the results shown in Figure 3 shows that, as the solution acidity increase, cobalt and
![]()
Figure 1. Price metal elements contained at 18650 lithium-ion batteries.
![]()
Figure 2. Process flow diagram of cobalt recovery by liquid leaching.
![]()
![]()
Figure 3. Effect of acid concentration on leaching rate and electrolytic cobalt photo.
aluminum solubility in the solution increases accordingly. With 10 mol/L sulfuric acid solution as an industrial leaching agent, the lithium-ion battery with the waste placed in 5 L beaker, heated to 70˚C, leaching 1 h, the reaction was completely dissolved, ICP testing the resulting solution composition shown in Table 1.
3.2. Neutralization Reaction to Remove Iron, Aluminum
Leaching with sodium carbonate as a neutralizing agent resulting solution was adjusted to pH 2 - 3, and heated to 90˚C, blast stirred solution of iron and aluminum precipitate. At the same time, also co-precipitation in the form of silicon is removed. After hydrolysis impurity solution composition shown in Table 2.
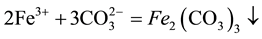 (1)
(1)
 (2)
(2)
3.3. Electrodeposition
Starting with the production of cobalt cathode pole piece, a titanium plate as an anode, the impurity resulting solution directly electrowinning current density of 240 A/m2, electrolyte temperature 55˚C - 60˚C, in return anolyte electrolysis and cleaning section the resulting electric cobalt surface roughness, the spectral analysis of its components, such as tables, Table 3, Figure 3 also lists the quality standards 1 and 1A # # electric cobalt electric cobalt [11] .
Table 3 shows full-immersion neutralized after hydrolysis direct electrowinning cobalt impurity, product quality can be made 1A electric cobalt standards. The electrolytic cobalt electricity obtained by the Formula (3)
![]()
Table 1. Content of each metal element in leaching solution.
![]()
Table 2. Content of each metal element in leaching solution after neutralization reaction to remove iron, aluminum.
![]()
Table 3. Cathode cobalt quality and criterion of GB6517-86.
obtain the current efficiency of the electrolysis process 90.01%.
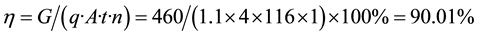 (3)
(3)
where: η―current efficiency, %; G―precipitation Electric Co Quality, g; q―cobalt electrochemical equivalent, 1.1 g Ah; A―current intensity, A; t―electrolysis cycle, h; n―cell number. Direct yields greater than 90% cobalt.
4. Conclusion
The use of sulfuric acid leaching-EW treatment of waste lithium 18605 lithium-ion battery technologies is simple. Cobalt leaching rate basically reached 100%, absolute yield of more than 90%. Leach solution after hydrolysis impurity can be directly electrowinning cobalt, simple process. We get 1A # electric cobalt comply GB6517- 86 by electrolyte. The current efficiency was 90.01%.