Determination of the Compound Biological Effectiveness (CBE) Factors Based on the ISHIYAMA-IMAHORI Deterministic Parsing Model with the Dynamic PET Technique ()
1. Introduction
Many types of pilot innovative accelerator-based neutron source for neutron capture therapy with lithium target were designed [1] - [3] and many inventions for the progressive power run-up were reported [4] [5] . In Japan, implemented deployment of accelerator-driven neutron source for Boron Neutron Capture Therapy (BNCT) was accomplished in 2014 in National Cancer Center, of which system was designed with the production of neutrons via threshold 7Li (p, n) 7Be reaction at 25 kW proton beam with energy of 2.5 MeV, which was designed to dovetail the narrow peak band resonance of lithium target and started its installation at middle of 2013. This BNCT device is expected to offer the potential for achieving the objects of which any treatment capable of sterilizing the primary tumor locally will result in a high probability of cure.
BNCT is a targeted radio-therapeutic modality used for the treatment of brain tumors and melanoma and a bimodal approach to cancer therapy. Before BNCT, Boron-10(10B)-enriched compounds are used to deliver 10B to tumors. Once tumor uptake of a given boron delivery agent relative to the surrounding normal tissues and blood has been maximized and then irradiation with low-energy neutron takes place. An alternative boron delivery agent, p-borononphenylalaine (BPA) instead of administration of the boron delivery agent borocaptate sodium (BSH), is being used together with mode deeply penetrating epithermal neutron beam [6] . BNCT is extensively reviewed in two recent articles [7] [8] and the targeting effectiveness of BNCT is dependent upon the preferential delivery of 10B to the primary tumor and its metastatic spread.
In defining the biological effects of the 10B(p, α)7Li neutron capture reaction relative to photons, the term compound biological effectiveness (CBE) factor was used as an alternative to RBE. Calculation of the CBE factor is similar to that of the RBE factor [9] . Equating the X-ray ED50 dose with a BNC dose (beam + BSH) that gives the same end point of a 50% incident of ulceration produces the following equation:
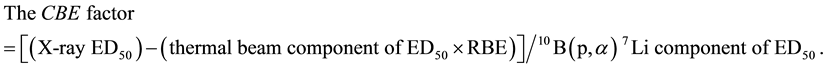
The CBE factors concerning to tumor, skin lung, liver [10] - [12] and oral mucosal tissues [13] are reported and prospect of actually using BNCT for the patients has been developing under the right circumstances. However, there is no theoretical unified explanation of the CBE factors for normal tissues and tumor, despite the fact that significance of high precision of the CBE factor evaluation is requested for the patients.
Recently, the authors proposed deterministic parsing model of CBE factors (ISHIYAMA-IMAHORI model) and applied to human tumor brain cases and derived good results dovetailed with empirical facts [14] [15] .
The purpose of the present investigation was to demonstrate the unified methodology for the evaluation of the CBE factors for normal tissues and tumor in BNCT.
2. Materials and Methods
2.1. 10B Concentration Measurement of BPA by Dynamic PET Technique
33 brain tumor patients (grade AII (8 patients), AIII (11) and GBM (14)) were given low dose (approximately ~ 100 μg/g) of intravenous radioactively-labeled 18F-BPA before BNCT and diagnosed cancer by Positron-Emis- sion-Tomography (PET) [16] . To obtain 10B concentration in a body, 18F-BPA was administrated to the patient by intravenous drip injection and PET inspection was performed in every 20 minutes to measure a change in 10B concentrations in tumor, normal and blood of the patient, respectively.
2.2. Mathematical Analysis Model for the 10B Concentration Data
After 10BPA administration, boron atoms are ingested into the cell model consisted of endoplasm and cell nucleus and Imahori [17] reported the kinetic analysis for brain tumor patients by using three-compartment rate constant (K1, k2 and k3) (Figure 1).
This model implied that the body injected 10BPA begins to rapidly up-taken into cancer cell group at the injection initial and eventually suppressed increase with increasing 10BPA-containing population.
As a function that can better represent this phenomenon, the sigmoid function are frequently applied as natural population increasing model. Accordingly, logistic function based on the sigmoid function was employed to analyze dynamic PET data. The logistic function in present study was defined as:
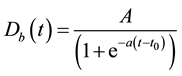 (1)
(1)
where Dbnormal and Dbtumor are 10B concentrations in tumor, normal tissues and time-dependent function. A, a and t0 in Equation (1) are constants, respectively. Iteration calculation technique was employed to obtain constants A, a and t0 in Equation (1) for normal tissue and tumor cases, respectively [15] .
3. Results
3.1. Dynamic PET Measurement for Normal Tissues and Tumor
Typical changes in 10B concentration in normal tissue, tumor and blood of a BGM patient are illustrated in the figure by 10BPA administration by intravenous and drip injection methods (Figure 2).
Sudden increase and peak in 10B concentration in blood, normal tissue and tissue were found just before intravenous injection of BPA administration. Whereas, the changes in 10BPA concentration after drip injection show modest slow changes in 10B concentration in normal tissues, tumor and blood, respectively (Figure 3).
These typical changes after 10BPA administration indicate compatibility to define saturation boron concentra- tion, Nmax and threshold of boron density, Nth for the determination of CBE factors by ISHIYAMA IMAHORI model [14] [15] as below:
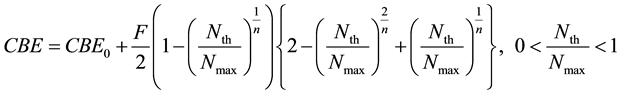 (2)
(2)
and this is because that we chose drip injection in present study.
![]()
Figure 1. Gjeddle-Patlak model using three-compartment rate constants (K1, k2 and k3).
![]() (a) (b)
(a) (b)
Figure 2. Typical change in 10B concentration in tumor, normal tissues and blood measured by dynamic PET technique with 10BPA administration by (a) intraveous injection and (b) drip injection methods.
![]()
Figure 3. Change in 10B concentration in blood, tumor and normal tissue measured by dynamic PET technique.
As for a typical change in 10B concentration in blood, tumor and normal tissue of a brain tumor patient (Grade IV), logistic function in Equation (1) was applied to these data. Compatibility of the function to normal tissue and tumor are provided in Figure 4 and Figure 5.
From these results, it is clear that very good data fitting curves of the logistic function to dynamic PET data were observed and each constant in Equation (1) are obtained in the tumor and normal tissue. These results are listed in Table 1.
3.2. Determination of the CBE Factor Depend on Boron Dose Level
To obtained threshold and saturation density of boron, Nth and Nmax in tumor and normal tissue from Equation (1), we defined Nth and Nmax as follows:
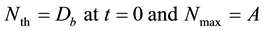 (3)
(3)
These values of Nth, Nmax and Nth/Nmax for normal tissue and tumor are listed in Table 2.
From these results, The CBE factors for normal tissue and tumor in a brain tumor patient were calculated by Equation (2) and these results are given in Table 3.
4. Discussions
4.1. The CBE Factors Estimations by the Severity of the Brain Tumor
The difference between the previous report [15] and this paper mainly lies in the definition of the Nth from the dynamic PET curves. From dynamic PET curves of 33 brain tumor patients contained of AII (8 patients), AII (11) and GBM (14) [16] , the CBE factors were calculated by the ISHIYAMA-IMAHORI model (Equation (2)) with definition of Nth/Nmax by Equations (1) and (3), and plotted in Figure 6. From these data, it is clearly
![]()
Figure 4. A change in 10B concentration in normal tissue measured by dynamic PET technique and logistic function.
![]()
Figure 5. A change in 10B concentration in tuomor measured by dynamic PET technique and logistic function.
![]()
Figure 6. CBE factors calculated by ISHIYAMA-IMAHORI model with for three grade of brain tumor patients as a function of Nth/Nmax.
![]()
Table 1. Constants in Equation (1) logistic function obtained for tumor and normal tissue.
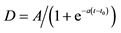 .
.
![]()
Table 2. The values of Nth and Nmax defined by Equation (3) for tumor and normal tissue.
Nth = D at t = 0; Nmax = A.
![]()
Table 3. The values of Nth/Nmax and CBE factor defined by Equation (2) for tumor and normal tissue.
categorized into three groups corresponding to the severity of the three grades and it can be given as individual numerical values for the individual patient in the same group.
4.2. Application of ISHIYAMA-IMAHORI Model to Other Cancer Affected Area Position
The ISHIYAMA-IMAHORI model can provide CBE factors about not only brain tumors, also cancer affected part of a different site by 18F-BPA dynamic PET measuring technique. Typical lung cancer that can be observed by PET with 18F-BPA was shown in Figure 7. There is a ling cancer (adenocarcinoma) in lower right lung field and the diaphragm just above, but no metastasis to the brain in this case.
From dynamic PET curve obtained in this case, Nth and Nmax values can be determined from temporal change in the color intensity of the target diseased part from the Equations (1) and (3), and the CBE factor in this case was evaluated as 6.35 from the ISHIYAMA-IMAHORI model.
4.3. Application of the Calculation Method and Its Clinical Significance
The charm of the BNCT treatment is that again and again for the same patients and their affected area is capable of irradiation treatment. Therefore, the cure of intractable cancer in a short time by BNCT treatment is not a dream. However, BNCT treatment at this stage is time-consuming due to the following reasons. Normally, cancer patients are given low doses of intravenous radioactively-labelled 18F-BPA before BNCT and diagnosed cancer by Positron-Emission-Tomography (PET). Physicians developed a treatment plan by BNCT based on PET diagnosis and then after administrates high dose of BPA to the patients.
So practical value of present research is that the diagnosis and treatment cycle can be achieved at the same time shorten with high accuracy.
Present research results, i.e. by 18F-BPA drip injection administration and dynamic PET measurement method, ISHIYAMA-IMAHORI model immediately provides a high-precision CBE factor and BNCT treatment for a kind of cancer and its severity in patients individual.
5. Conclusions
ISHIYAMA-IMAHORI model below immediately provides a high-precision CBE factor and BNCT treatment for a kind of cancer and its severity in patients’ individual by 18F-BPA drip injection administration and dynamic PET measurement method
![]()
Figure 7. Typical lung cancer case that can be observed by PET with 18F-BPA.
![]()
And Nth/Nmax is obtained by the flowing logistic function
![]()
where Bb is 10B concentration in tumor and normal tissue, and A, a and t0 are constants.