1. Introduction
Polycarbonate (PC) is a very durable material. It has high impact-resistance, but a low scratch-resistance. It can undergo large plastic deformations. The light transmission characteristics of polycarbonate are even better than many kinds of glasses. It has characteristics quite like those of polymethylmethacrylate (PMMA, acrylic), but PC is stronger and usable in a wider temperature range. It is utilized in making eyewear lenses and exterior automotive components. Being good electrical insulator and having heat resistant and flame retardant properties, it is used in various products associated with electrical and telecommunications hardware. The polycarbonates are generally those polymers wherein dihydric or polyhydric phenols are joined through carbonate linkages and are considered to toughest of all thermoplastics. Its scientific applications include track recording properties in neutron dosimetry, heavy ion and nuclear physics and detection of gamma rays. The optical, chemical, electrical and structural properties of this polymer can be tailored in a tunable by exposing it to ionizing radiation.
The exposure of ionizing radiations like energetic heavy ions, X-rays/γ-rays etc. to polymeric substances may be employed frequently to tailor their properties. The exposure of ion beam may create ion tracks in the polymers [1] . In addition to this, the ionizing radiations may also produce random fracture of main chain and braking of original bonds giving rise to the formation of free radicals, double/triple bonds, cross linking [2] - [8] , carbon cluster formation [9] [10] and evolution of ionic species and gaseous components etc. [11] [12] .
Crosslinking of bisphenol-A polycarbonate in the presence of bisphenol-A dimethacrylate and triallyl cynurate as a result of γ-irradiation was reported by Aliev et al. [4] . Srivastava et al. [5] examined the response of 100 MeV silicon beam on chemical, optical and thermal changes of polycarbonate. A slight red shift in the optical absorption spectra and formation of phenolic bond was observed. The structural response of 3 MeV proton irradiation upon Makrofol-DE PC was studied by Singh et al. [6] up to 1013 ions/cm2. The material was found to suffer severe degradation and structural modifications due to bond breakage at the highest fluence. Rajesh et al. [7] reported a red shift in the UV-Vis spectra of 70 MeV C+5 bombarded Macrofol PC. The structural modifications via FTIR spectroscopy were also reported at higher fluences. The optical and electrical properties of PC after He and Ar ion irradiation were studied by Radwan et al. [8] . A decrease in the optical band gap and an increase in electrical conductivity were reported. Yap et al. [9] reported the appearance of yellow-brown material having optical properties like amorphous hydrogenated carbon when PC was implanted with H ions. While the Ar ion implantation produced a material that showed optical properties like evaporated amorphous carbon. The Raman spectroscopy of 1 MeV Ag+ ions irradiated PC was studied by Bahniwal et al. [10] and pointed out the formation of carbonaceous graphite-like layer containing a network of graphite clusters. Navarro et al. [11] showed that PC irradiation was accompanied with the preferential release of carbon monoxide followed by minor production of hydrogen, carbon dioxide and methane.
The present worked out problem was motivated to investigate physical and chemical response of γ-radiation on polycarbonate polymer, especially the modification in the optical, chemical and structural properties through UV/Vis and FTIR spectrometry.
2. Experimental Details
Test samples of about 30 × 30 mm size were cut from flat sheets of polycarbonate of thickness 250 micron. These samples were placed in polyethylene sachets and exposed to γ-radiation from a 60Co γ-source at BARC, Mumbai at a dose rate of 2.76 kGy/h in air at room temperature. The samples were irradiated for various time periods to get the total accumulated doses of γ-radiation as 1, 2, 5, 10, 20, 50, 117, 199, 500 and 800 kGy respectively.
The optical absorbance for each sample were assessed in the wavelength range 190 to 900 nm at room temperature with the help of Shimadzu Double Beam Double Monochromator UV-Visible spectrophotometer (UV- 2550) with a resolution of 0.05 nm at Kurukshetra Uuniversity, Kurukshetra. The air was taken as reference. From these data the optical constants such as absorption edge, optical band gap (both direct and indirect), Urbach energy were determined.
The FTIR spectroscopy was done using Perkin-Elmer spectrophotometer “Spectrum RX-I” in the range of 400 cm−1 - 4000 cm−1 with a resolution of 2 cm−1, at Sophisticated Analytical Instrumentation Facility, Punjab University, Chandigarh.
3. Results and Discussions
The pristine polycarbonate samples were colorless. The visual observations of irradiated films indicate that the polycarbonate samples above 50 kGy turn yellow with the increase of γ-radiation dose. The yellowish ness of polycarbonate increases with the increase of γ-radiation dose. The UV-Visible absorption spectra of pristine and γ-irradiated polycarbonate films with doses of 20, 50, 117, 199, 500 and 800 kGy in the wavelength range 200 to 600 nm are shown in Figure 1.
It is clear from the Figure 1 that above 450 nm, no significant absorption occurs and a flat plateau region is observed for all radiation doses. Enhancement in the absorbance was observed with the increase of incident γ-radiation dose. The absorption bands between 200 - 400 nm become broad and with the increasing dose of γ-radiation and the tail of the absorption spectra shift towards visible region from UV region. The above observations can arise due to the consequence of the formation of new chemical species caused as a result of energy deposition from the incident γ-rays [2] . This energy deposition may lead to the excitation and ionization lading to the breaking of original bonds, main chain scission, radical formation, cross linking [4] etc. This in turn results in the formation of double/triple bonds, conjugation of bonds [7] , formation of carbon clusters [10] etc. The absorption bands in the investigated range are associated to the π - π* electronic transition [13] - [15] . This excitation of π electron requires small energy and hence transition of this type occurs at longer wavelengths.
4. Modifications in Optical Energy Band Gap
The study of optical absorption spectra can be used as one of the methods in understanding the band structure and energy gap of amorphous materials. This can be utilized to measure the radiation-induced absorption, the optical energy band gap and the width of the absorption tail. Any change in these parameters is a sign of modification in the structure of the material. Tauc’s [16] analysis gives us the opportunity of dividing absorption edge into three different regions as follows: 1) a week absorption tail arising from defects and impurities, 2) the exponential region, that is strongly connected to the structural randomness of the system, and 3) high absorption region, which gives optical energy band gap. Davis and Shilliday [17] showed that near the fundamental band edge, both direct and indirect transitions occur. The Tauc’s expression [18] for the determination of optical energy band gap was discussed in a broad-spectrum by Mott and Devis [19] and Thutupalli and Tomlin [20] . According to them the direct and indirect band gap (Egd and Egi respectively), of a semiconductor are related to the absorbance (α) as follows:
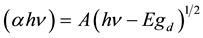
![]()
where hν is the photon energy, and A and B are constants.
![]()
Figure 1. UV-Vis absorption spectra of pristine and irradiated polycarbonate films.
These relations have been reported to hold good for polymers also [7] [12] .
For the determination of nature and width of absorption edge, direct and indirect band gaps α, 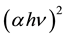 and
and  were plotted as a function of photon energy (hν) respectively. The linear portion of the fundamental absorption tail of the UV-visible spectra was extrapolated as shown in Figures 2-4. The absorption edge values were obtained by extrapolating the linear portions of α versus photon energy (hν) curves to zero ordinate as shown in Figure 2.
were plotted as a function of photon energy (hν) respectively. The linear portion of the fundamental absorption tail of the UV-visible spectra was extrapolated as shown in Figures 2-4. The absorption edge values were obtained by extrapolating the linear portions of α versus photon energy (hν) curves to zero ordinate as shown in Figure 2.
The direct band gap energies were also determined by extrapolating the linear portions of the 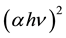 vs. hν (photon energy) curves, from the intercepts of the least squares fitted lines on hν axis as shown in Figure 3.
vs. hν (photon energy) curves, from the intercepts of the least squares fitted lines on hν axis as shown in Figure 3.
The indirect band gaps were obtained from the 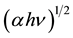 versus (hν) photon energy curves in a similar manner as shown in Figure 4.
versus (hν) photon energy curves in a similar manner as shown in Figure 4.
![]()
Figure 2. Plots for absorption edge in pristine and irradiated polycarbonate.
![]()
Figure 3. Plots for direct band gap (eV) in pristine and irradiated polycarbonate.
![]()
Figure 4. Plots for indirect band gap (eV) in pristine and irradiated polycarbonate.
The Urbac tail of the absorption edge is usually attributed to the optical electronic transitions between the protracted states and the near edge localized states. The development of localized states with energies at the boundaries of the energy gap is one of the effects of the structural disorder on the electronic structure of amor- phous material. This is why Urbach energy is considered to be a measure of the of the degree of disorder [21] [22]
In the exponential edge region, the absorption coefficient 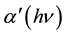 can be described by the Urbach’s exponential law [23] as follows
can be described by the Urbach’s exponential law [23] as follows
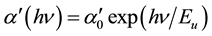
Here, 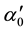 is a constant and Eu is called Urbach energy, characterizing the slope of the exponential edge region and can be interpreted as the width of the band tails of the localized states. Since envelope method is not valid in the strong absorption region, the calculation of the absorption coefficient in this region can be done with help of the following relation
is a constant and Eu is called Urbach energy, characterizing the slope of the exponential edge region and can be interpreted as the width of the band tails of the localized states. Since envelope method is not valid in the strong absorption region, the calculation of the absorption coefficient in this region can be done with help of the following relation
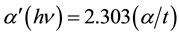
In order to determine Urbach energy Eu, 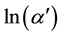 was plotted as a function of photon energy as shown in Figure 5. Inverse of the slope of the fitted lines give Urbach energies.
was plotted as a function of photon energy as shown in Figure 5. Inverse of the slope of the fitted lines give Urbach energies.
These values along with their errors have been determined for pristine and irradiated polycarbonate samples to different doses of γ-radiation. The values of band edge, direct and indirect band gaps Urbach energy Eu obtained from Figures 2-5 are listed in Table 1.
The magnitude of regression coefficient, have been found to be ≈0.999 for the fitted lines, shown in Figures [2] -[5] for the determination of absorption edge, direct band gap, indirect band gap and Urbach energy. This clearly indicates the simultaneous existence of direct and indirect band gap in polycarbonate polymer with decreasing tendency with the increasing γ-radiation dose. The values of indirect band gap have been found to be lower than the corresponding values of the direct band gap in the polycarbonate polymer subjected to γ-radiation. The values of Urbach Energy of irradiated PC Samples were found to increase with the increasing dose of γ-radiation.
5. Carbon Atoms in Conjugation
From the abscissas of 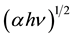 versus photon energy (hν) curves plotted in Figure 4 of irradiated polycarbonate
versus photon energy (hν) curves plotted in Figure 4 of irradiated polycarbonate
![]()
Figure 5. Plots for Urbach energy (eV) in pristine and irradiated polycarbonate.
![]()
Table 1. Absorption edge position, direct and indirect optical band gaps and Urbach energy of pristine and irradiated polycarbonate films.
the number of carbon atoms per conjugation length has been calculated using Equation (1) given by Robertson’s and O’reilly [7] [24] as
![]() (1)
(1)
where, N denotes the number of carbon atoms per conjugation length for a linear chain polymer. 2β is the band structure energy of a pair of neighboring π sites. The value of β is taken to be −2.9 eV as it is associated with π - π* optical transition in C=C linkage [7] . Eg was taken as the lower value of band gap i.e. the indirect energy band gap. The number of carbon atoms in conjugation (N) calculated from above equation in the present work at different doses of γ-radiation is given in Table 2.
6. Carbon Cluster Size
Fink et al. [3] pointed out that the Robertson’s and O’reilly equation underestimates the cluster size in the irradiated polymers. They have accordingly assumed the structure of the clusters to be buckministerfullerene, i.e.
![]()
Table 2. The number of carbon atoms in conjugation calculated from Equation (1) along with the carbon cluster size calculated from Equation (2).
comprising of C60 rings instead of C6, and arrived at the relation
![]()
where M is the number of carbon atoms per cluster. The above relation can be used to obtain the number of carbon atoms per cluster in irradiated polycarbonate.
Thus,
![]() (2)
(2)
7. Structural Modifications
The nature of the chemical bonding and the structural modifications due to the energy deposition from the incident irradiation in the polymers can be studied by characterizing the vibrational modes obtained from infrared spectroscopy of pristine and irradiated samples [25] -[27] . The FTIR spectra of Pristine and the γ-irradiated PC films over the wave no. range 400 - 4000 cm−1 are shown in Figure 6. The transmission FTIR spectrum of the Pristine PC films showed (a) 1590 cm−1; C-C multiple bond stretching of aromatic ring (b) a band about 1625 - 1650 cm−1: due to C=C stretching vibrations (c) 1760 cm−1; due to C=O stretching vibration (d) 2860 - 3000 cm−1; C-H vibrations of CH2 and CH3 groups (e) 3040 cm−1; C-H vibrations of aromatic compound and (f) a band about 3540 cm−1 due to the presence of (−OH) functional group. The FTIR spectra of PC films irradiated to different doses of γ-radiation indicate a slight increase in the transmission intensity of the bands corresponding to C-H vibrations along with C=O stretching while a slight decrease in the intensity of the band attributed to −OH group with the broadening of this band. This may be an indicative of the dissociation of carbonate linkage with probable elimination of carbon dioxide/carbon monoxide [7] [11] indicated by the further enhancement in the absorption intensity at −C=C− band and generation of more −OH. The generation of hydroxyl group may take place due to the absorption of oxygen/H2O from the atmosphere during irradiation or post-irradiation circumstances. The broadening of −OH band is an indicative of occurrence of intermolecular bonding. Navarro- González [11] observed the formation of phenol after the Pyrolysis of γ-irradiated bisphenol-A polycarbonate.
8. Conclusion
The γ-irradiated PC films show yellowing of the samples with the increase of γ absorbed dose. The structural modifications that are indicated by the intensity patterns of various bands in the FTIR spectra of pristine and irradiated PC samples suggest chain scissoring at carbonate site with probable elimination of carbon di/monoxide and increase in hydroxyl formation. It can also be concluded from the UV-Vis spectrometric studies that the UV-Vis spectra of virgin and irradiated polycarbonate reveal the broadening of bands and a red shift of absorption tails with the increase of γ-irradiation dose. Coexistence of direct and indirect band gaps simultaneously was observed. The indirect band gap values were found lower as compared to the corresponding values of direct
![]()
Figure 6. FTIR spectra of pristine and irradiated polycarbonate.
band gap in the pristine and γ-irradiated polycarbonate. Both types of the optical band gap energies have decreasing tendency with the increasing γ-radiation dose. The increase in conjugation length −C=C− and carbon cluster size can be correlated with the optical energy band gap. The increase in Urbach energy with progressive γ-radiation dose indicates increase in the degree of structural disorder as indicated by the increase in carbon cluster size with the increase of γ absorbed dose.
NOTES
*Corresponding author.