Different Effects of Malate on the Activities of Photosystem II in Detached Leaves of Maize and Tobacco ()
1. Introduction
C4 plants possess elevated photosynthetic ability, water use efficiency and nitrogen use efficiency compared with C3 plants. They have better yield performance under high light intensity, high temperature and drought. Therefore, an effort is currently underway to incorporate these characteristics into C3 plants by genetic engineering [1] - [7] . However, most such attempts have not achieved the expected goals [5] [8] . In typical C4 plants, mesophyll cells (MC) and vascular bundle sheath cells (BSC) in the leaves are arranged to form the Kranz anatomy around vascular tissues. These two types of cells are different not only in structure but also in function. In MC chloroplasts, the activity of photosystem II (PS II) is normal, but the activity of ribulose bisphosphate carboxylase/oxygenase (Rubisco) is lower. On the other hand, in BSC chloroplasts, the activity of PS II is lower but the activity of Rubisco is higher [9] -[11] . For C3 plants, leaves have not evolved the structure and function like C4 plants. To this day, we know less about the regulation of the differentiation of chloroplast in structure and function in BSC and MC in C4 plants. Several reports showed that the operation and efficiency of C4 photosynthetic cycle were closely related to the stage of leaves. Maize is a C4 plant of NADP-dependent malic enzyme (NADP-ME) type. Its leaves of from 1st to 3rd run the C4 cycle with lower efficiency, and with lower activity of C4 photosynthetic enzymes in MC and higher activity of PS II in BSC [12] [13] . The 4th leaf completes the differentiation of MC and BSC, with higher rate of C4 photosynthesis. Tobacco is a C3 plant which photosynthesizes in MC and not in BSC. Malate is the first stable product after CO2 is fixed in maize, transferring CO2 and the reducing equivalent from MC to BSC chloroplasts and affecting the redox state of BSC. The input of malate is paralleled with the changes in the structure and function of chloroplast in BSC. This suggests that the malate might contribute to the regulation in loss of grana and deficiency in PS II activity in BSC chloroplast of C4 plants. The aim of this experiment is to investigate the effect of exogenous malate on the activity of PS II in C4 plant maize and C3 plant tobacco. The discs from 5th leaves of maize and 10th leaves of tobacco were treated with malate of 50 μM and 100 μM, and the chlorophyll fluorescence parameters were measured.
2. Materials and Methods
2.1. Plant Growth and Treatments
Maize hybrid Zhengdan 958 and tobacco K326 were grown in growth chamber, under a 14 h photoperiod (500 μmol∙m−2∙s−1 PFD) and a day/night regime of 26˚C/22˚C. The 5th fully expanded leaves of maize and the 10th fully expanded leaves of tobacco were used to sample. Six discs of 1cm diameter were taken from the middle section of leaves. The discs were infiltrated under vacuum for 60 min with malate and tartrate solution (0, 50, 100 μM), containing 10 mM KCl and 0.1 mM Mes/BTP, pH 5.5. Tartrate is a structural analog of malate.
2.2. Measurement of Chlorophyll Fluorescence
The discs were subjected to dark for 15 min and then exposed to 3000 μmol∙m−2∙s−1 PFD generated by Handy- PEA (Hansatech, UK) for 1 s [14] . According to the JIP-test [15] [16] , the following parameters were obtained: 1) FO: the initial fluorescence yield; 2) FM: the maximum fluorescence; 3) FV: the variable fluorescence; 4) FV/FM: maximal efficiency of PS II photochemistry; 5) RC/CS: the number of active PS II reaction center (RC) per excited cross section (CS); 6) ABS/RC: absorption flux per RC; 7) TRO/RC: trapped energy flux per RC; 8) ETO/RC: the electron transfer efficiency of active PS II reaction center; 9) WK: the normalized relative variable fluorescence at the K step,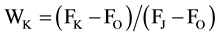 .
.
2.3. Measurement of Photosynthetic Oxygen Evolution
The discs were subjected to light of 500 μmol∙m−2∙s−1 for 30 min at 25˚C before measurement. Photosynthetic oxygen evolution rate was measured with a Clark-type oxygen electrode (Chlorolab 2, Hansatech) at 500 μmol∙ m−2∙s−1 at 25˚C. The measurement was conducted in 0.4 mM NaHCO3. Gross oxygen evolution is equal to the sum of net oxygen evolution and dark respiration.
3. Results
3.1. Changes in FO, FM, FV and FV/FM in Maize and Tobacoo
FO is the amount of fluorescence release when all PS II RCs are open, affected by ratio of active PS II reaction center (RC)/light-harvesting complex II (LHC II) and the efficiency of excitation energy transfer to PS II RC. The efficiency of excitation energy flow to RC depends on the structure of LHCII and the connection between LHCII and RC. FM is the amount of fluorescence release when all PS II RCs are closed, representing the maximal light absorption potential. This parameter is related to the total amount of light harvesting pigments. In malate treatment, tobacco has no significant difference in FO and FM, but maize has a significant increase in FO and mild decline in FM (Table 1). The decline in FM can be explained by the decrease in light-harvesting pigment caused by malate treatment. The effect of malate on FO is possibly caused by the change in the ratio RC/LHC II, the structure of LHC II and the connection between LHC II and RC. In fact, in this experiment, RC and FM were reduced in malate solution (Figure 1 and Table 1). Therefore, the increase in FO in this study is mainly attributed to the change in structure of light-harvesting complex and the connection between LHC II and RC.
FV is the difference between FM and FO, reflecting the photochemical reaction potential and affected by the amount of RC, the activity of photochemical reaction and the efficiency of electron transfer. In tobacco, FV has no significant difference under 50 μM malate and mild decline under 100 μM malate. In maize, there was a significant decrease in FV (Table 1). The decline of FV is not only caused by the rise in FO, but also by the drop in FM.
FV/FM shows the maximal efficiency of PS II photochemistry. Under malate treatment, tobacco has a slight variety in FV/FM. But maize has a remarkable decrease under 100 μM malate (Table 1), which is mainly attributed to the reduction in FV. The reduction in FV/FM mainly resulted from the increase in FO. This means that the increase in Mal metabolism reduced PS II efficiency in maize leaves.
Comparing the same basic flourescence indices between tobacco and maize, besides FV/FM, the other indices of tobacco are all lower than maize. The high level of FV/FM in tobacco probably due to its low level of FM.
Under tartrate treatment, these four basic flourescence indices didn’t show significant difference in both tobacco and maize discs.
3.2. Changes in RC/CS and ETO/CS in Maize and Tobacoo
RC/CS is the amount of QA-reducing PS II RC per excited cross section (CS), that is, the number of active PS II RC. In tobacco, RC/CS didn’t show significant difference under malate treatment. Malate treated maize discs showed a remarkable decrease in RC/CS (Figure 1(a)), which suggested that exogenous malate has an effect of inactivating PS II RC. Because the activity of RC is affected by the structure of PS II complex, malate treatment must have changed the structure of PS II RC. Besides 100 μM malate treatment, RC/CS in tobacco were all lower than in maize.
ETO/CS describes the electron transport flux of RC per excited cross section (CS), affected by RC/CS, the ability of RC to reduce QB and the efficiency of electron transfer chain. In this study, no remarkable difference were observed in tobacoo discs. Malate treatment significantly decreased ETO/CS in maize discs (Figure 1(b)), which might be resulted from the decline in RC/CS (Figure 1(a)) and the QB-reducing ability of RC (Figure 2(c)). ETO/CS in tobacco were all lower than in maize.
Under tartrate treatment, RC/CS and ETO/CS didn’t show significant difference in both tobacco and maize discs.
3.3. Changes in ABS/RC, TRO/RC, ETO/RC and WK in Maize and Tobacoo
ABS/RC is the maximum light absorption per RC, reflecting the ratio of light harvesting pigment to RC. In tobacco, ABC/RC didn’t show significant change under malate treatment (Figure 2(a)). In maize, the increase in ABS/RC in malate solution (Figure 2(a)) suggested that Mal treatment altered the ratio of ABS/RC. Because the FM was decreased by Mal (Table 1), the rise in ABS/RC ratio must be attributed to the decline in RC amount. ABS/RC in tobacco were all higher than in maize.
TRO/RC describes the trapped excitation energy per RC, representing the potential of excitation energy attained per RC. In tobacco, TRO/RC didn’t show significant change under malate treatment (Figure 2(b)). In maize, the increase in TRO/RC in malate solution (Figure 2(b)) is attributed to the decline in RC amount. This means that, compared with the control, each RC can attain more supply of excitation energy in malate treatment leaves. TRO/RC in tobacco was all higher than in maize.
ETO/RC is the amount of excitation energy used per RC in photosynthetic electron transfer, affected by ability of RC to reduce QB and the efficiency of electron transfer chain. In this study, ETO/RC describes the electron
![]()
Table 1. Changes in basic flourescence indices FO, FV, FM and FV/FM in tobacco and maize discs treated with malate and tartrate (50, 100 μM).
Single asterisks (*) and double asterisks (**) indicate the significance of difference at P < 0.05 and P < 0.01 levels, respectively, by F test when compared with that in control plants. Values are means ± S.D. (n = 5).
![]()
Figure 1. Changes in RC/CS and ETo/CS in tobacco and maize discs treated with malate and tartrate (50, 100 μM). (a) RC/CS is the amount of QA-reducing PS II RC per excited cross section (CS); (b) ETo/CS describes the electron transport flux of RC per excited cross section (CS). Single asterisks (*) and double asterisks (**) indicate the significance of difference at P < 0.05 and P < 0.01 levels, respectively, by F test when compared with that in control plants. Values are means ± S.D. (n = 5).
transport flux per RC. In tobacco, it didn’t show significant change under malate treatment (Figure 2(c)). In maize, malate treatment significantly decreased ETO/RC (Figure 2(c)), which showed that the QB-reducing ability of RC was inhibited and the flow of electron transfer was blocked. If chloroplasts in treatment leaves acquired CO2 from malate as carbon source to operate Calvin Cycle, also accepted one molecule of NADPH. This means that Calvin Cycle only needs one NADP generated by linear electron transfer chain to reduce CO2. Consequently, linear electron transfer was blocked. ETO/RC in tobacco was all lower than in maize.
WK is the variable fluorescence at K point of JIT curve, equal to the ratio of variable fluorescence FV to the amplitude FJ-FO, representing the severity of oxygen-evolving complex (OEC) damage [17] . WK didn’t show significant change under malate treatment in tobacco but it increased in malate-treated maize leaf discs (Figure 3). The results showed that malate promoted the change in structure of OEC in maize leaf discs. This will reduce the activity of PS II, even inactivate the PS II. The significant rise in FO can be partially explained by the increasing WK. Besides 100 μM malate treatment, WK in tobacco were all higher than in maize.
Under tartrate treatment, ABS/RC, TRO/RC, ETO/RC and WK didn’t show significant difference in both tobacco and maize discs.
3.4. Oxygen Evolution in Maize and Tobacoo
The O2 evolution reaction is conducted in the O2-evolving complex of PS II in chloroplasts. As the primary electron donor, water is oxidized and releases dioxygen and protons during linear photosynthetic electron transfer. The electron released from water is transported to NADP to produce NADPH. Thus, O2 evolution can indicate the activity of PS II OEC and the electron transport activity of PS II. Figure 4 shows the O2 evolution of malate-treated discs, compared with the controls, the O2 evolution didn’t show remarkable change in malate-treated tobacco leaf discs. But it declined by 23.8% and 28.3% treated with 50 and 100 μM malate in maize leaf discs. These declines are consistent with the rise in WK and decrease in RC/CS and ETO/CS. O2 evolution in tobacco was all lower than in maize.
Under tartrate treatment, O2 evolution didn’t show significant difference in both tobacco and maize discs.
![]()
Figure 2. Changes in ABS/RC, TRo/RC and ETo/RC in tobacco and maize discs treated with malate and tartrate (50, 100 μM). (a) ABS/RC is the absorption flux per active PS II reaction center (RC); (b) TRo/RC the trapped energy flux per RC; (c) ETo/RC the electron transfer efficiency per RC. Single asterisks (*) and double asterisks (**) indicate the significance of difference at P < 0.05 and P < 0.01 levels, respectively, by F test when compared with that in control plants. Values are means ± S.D. (n = 5).
![]()
Figure 3. Changes in the normalized relative variable fluorescence at the K step (Wk) in tobacco and maize discs treated with malate and tartrate (50, 100 μM). Single asterisks (*) and double asterisks (**) indicate the significance of difference at P < 0.05 and P < 0.01 levels, respectively, by F test when compared with that in control plants. Values are means ± S.D. (n = 5).
![]()
Figure 4. Changes in O2 evolution of PS II in tobacco and maize discs treated with malate and tartrate (0, 50, 100 μM). Single asterisks (*) and double asterisks (**) indicate the significance of difference at P < 0.05 and P < 0.01 levels, respectively, by F test when compared with that in control plants. Values are means ± S.D. (n = 5).
4. Discussions
PS II supercomplex consists of reaction center, oxygen-evolving complex and light-harvesting complex. The activity of PS II depends on its structure, including the structure of each complex and the connection between complexes. The factors that disrupt the architecture of supercomplex will decrease the PS II activity. In this experiment, malate treatment inhibited the potential of trapping light energy (FV) and reduction ability of reducing QB (ETO/CS). Further analysis show that these changes are mainly resulted from the increase in FO and inactivation of PS II RC. FO represents the amount of useless energy absorbed by photosynthetic pigments. The increase in FO means that the efficiency of excitation energy transfer to PS II RC decreases. In this study the rise in FO is caused by the reduction in the ratio RC/LHC II (Figure 1) and the disrupt in the structure of OEC (WK) (Figure 3). The inactivation of RC may be mainly attributed to the injury of OEC. Possibly the connection between LHCII and RC is affected by malate. However, until now, there is no any suitable parameter in fluorescence JIT cure to describe this change in connection between PS II complexes. In addition, the tartrate (a structural analog of malate) treatment was not affected PS II in both maize and tobacco. It confirmed that activities of PS II were affected directly by exogenous malate but not pH or other indirect factor.
NADP is the terminal acceptor of linear electron transfer. As a result, the efficiency of transfer is affected by NADP/NADPH. In malate treated leaves, a large amount of exogenous malate is imported into chloroplasts and decarboxylated by NADP-ME to produce CO2, NADPH and pyruvate. This reaction will add NADPH into chloroplasts and decrease the NADP/NADPH ratio. The reduction of NADP molecules will result in the block of linear electron transfer. The pyruvate produced by malate decarboxylation may also play a role in change in the activity and structure of PS II in malate-treated leaves. We conclude that exogenous malate regulates the activity and structure of PS II in C4 plant maize.
The operation of C4 cycle in NADP-ME type C4 plants depends on four key enzymes. For C4 cycle, phosphoenolpyruvate carboxylase (PEPC), NADP-malate dehydrogenase (NADP-MDH), NADP-ME and pyruvate (PPDK), and regulated by the transporters of malate in cell plasma and chloroplast membrane. Malate treatments did not impose a significant effect on the activity and structure of PS II in tobacco detached leaves in this study. This may be attributed to the deficiency in NADP-ME and malate transporters with high capacity. Hudspeth et al. (1992) [1] reported that the transformation of C4-PEPC into tobacco increased the content of malate, but not promoted the photosynthetic rate. The overexpression of maize C4 NADP-ME in rice enhanced the decarboxylation of malate [3] [4] . We suppose that NADP-ME is a pivotal enzyme for the participation of malate in photosynthetic cycle in chloroplasts in tobacco. Because, in this experiment, leaf discs were treated with malate only for 60 min, we cannot deny that malate may impose effect on PS II activity and structure during a longer term treatment.
5. Conclusion
All in all, we conclude that exogenous malate regulates the activity and structure of PS II in C4 plant maize. No significant changes in the activity of PS II were observed in malate-treated C3 plant tobacco. It is suggested that the short term malate treatment will inhibit PS II of leaves which have C4 anatomy and C4 enzymes.
Acknowledgements
Supported by the National Natural Science Foundation of China (31000673, 31370283, 31201141 and 31170232), Technology Pillar Program of Liaoning Province, China (2015103001), Postdoctoral Science Foundation of China (2014M561097), Ministry of National Education Doctoral Fund of China (20102103110001), Science and Technology Development of Liaoning Province, China (2014208001).
NOTES
*Corresponding authors.