1. Introduction
Integrated Pest Management (IPM) is an effective and environmentally approach to pest management that relies on a combination of chemical and biological control methods. Of particular interest, parasitoids of the genus Trichogramma, which are egg-parasitoids in the family Trichogrammatidae, have been reported to be efficient in regulating populations of lepidopteran pests in agricultural cropping systems [1] [2] . Among main lepidopteran insect pest of olive tree, the olive moth Prays oleae Bern (Lepidoptera, Hyponomeutidae) is the most abundant throughout the Mediterranean and the Black Sea, the Middle East and the Canary Islands [3] . In Tunisia, the damage caused by P. oleae can reduce olive production by 50% [4] . As a consequence, efforts to develop biological control methods in IPM programs were initiated through the release of egg parasitoids and use of pheromones. However, release and integration issues that are considered important for the development of a successful IPM include important factors such as the pesticide choice and the timing of application [5] . In fact, Trichogramma is generally sensitive to pesticides [6] and most chemical products can be immediately toxic (contact toxicity) or it can persist (toxicity of dried residue) [7] [8] . Moreover, contact with pesticides at the less susceptible life stage (parasitoids within hosts) can cause prolonged development time of immature stages and reduced emergence from parasitized eggs [9] . Accordingly, this is important to determine the compatibility of parasitoids with pesticides for IPM programs.
Saccharopolyspora spinosa [10] or spinosad is a product classified as an environmentally and toxicologically reduced risk material [11] . Spinosad-based products have been registered in more than 30 countries for control of pest: Lepidoptera, Diptera, some Coleoptera, ants and thrips [12] . As a biorational pesticide, spinosad now represents an important option for pest control in a growing number crops produced under systems of integrated pest management (IPM) [13] . A recent review of predator and parasitoid susceptibility to spinosad concluded that this product represented one of the most judicious insecticides available for the conservation of predator populations [14] . However, the majority of laboratory and field studies report moderately harmful or harmful effects on populations of hymenopteran parasitoids [15] - [17] . Spinosad is commercially available under the name of Tracer 240® and is used against the first and second generations of P. oleae in most olive groves in Tunisia. However larval control of the first and the second generation of P. oleae usually rely on insecticides when the damage threshold reaches 5% for flowers and 20% for fruits. Pesticide persistence and mobility are influenced by the properties of the pesticide as well as by the soil environment, site conditions, weather and application methods. Therefore, information on degradation rate also helps to assess and predict the environmental behaviour of the pesticide.
Primary insecticide used is the pyrethroid, deltamethrin (Decis®), which is valued for its residual activity, persistence and high toxicity to terrestrial invertebrates [18] . Generally, the second generation is difficult to manage with insecticides because larvae bore into the fruit immediately after emerging from the eggs [19] . Other control measures are than needed and a potential alternative strategy is the release of Trichogramma species. In fact, this is a way to inhibit first larval stage of P. oleae on fruits [5] . However, one factor that may inhibit the ability of Trichogramma species to regulate pest population is the toxic insecticide residues.
Today, more than studying the toxic effect of pesticides on pests, there is a trend to evaluate their innocuousness towards natural enemies; These residues may be found in the soil, groundwater or the sediment of waterways and may also be found in vertebrates/invertebrates associated with the treated area, through direct contact or food chain effects [20] .
The selection of the most adequate parasitoid species is vital for any program of biological control [21] . It is important to select the appropriate Trichogramma species that are most adapted to the local field conditions for biolo- gical control programs. In fact, according to [22] , native Trichogramma species, which are generally adapted to environment, are better biological control agents. Moreover, quality measures that can be associated with the effectiveness of Trichogramma species to control the target pest are attributed mainly to the parasitism viability and to the developmental time from egg to adult emergence [23] . Therefore, the present study was designed to determine the side-effects of two insecticides deltamethrin (Decis®) and spinosad (Tracer 240®) on three native Trichogramma species T. oleae, T. cacoeciae and T. bourarachae [24] and to discuss their possible use in control programs against the second generation of P. oleae in olive grove areas where biological control has limited use.
2. Materials and Methods
2.1. Rearing of Trichogramma Parasitoid
Trichogramma species: T. oleae, T. cacoeciae and T. bourarachae used for the experiment were reared under laboratory conditions on Ephestia kuehniella eggs. Glued cardboard squares (1 cm2), each with approximately 250 E. kuehniella eggs, were presented for 24 hours to recently emerged Trichogramma females (20 females per card) in glass tubes ( 6 cm × 1.5 cm ) at 20˚C, 60% RH and 16L: 8D. The cards with a parasitism rate close to 100% were than transferred to other glass tubes and maintained until pupation (192 hours).
2.2. Treatment of Olive Trees with Insecticide
Two years young olive trees maintained in greenhouse at 25˚C, 70% RH were used for the sampling. Five olive trees per insecticide were sprayed till run-off with a hand sprayer at the same day ( 3 litres of solution per tree) and an eleventh tree sprayed with water for the control. Treatments were applied according to the recommended label rate for control of P. oleae. The insecticides tested were: deltamethrin at 3.75 grams∙ha−1 (Decis® 100 milliliters∙ha−1) and spinosad at 24 grams∙ha−1 (Tracer® 20 milliliters∙ha−1).
Olive leaves were collected at 3, 10, 17, 24 and 31 after olive trees spraying and used for exposure tests of parasitoid pupae at intervals after treatment.
2.3. Exposure Tests of Parasitoid Pupae at Intervals after Treatment
In each time after the treatment (at 3, 10, 17, 24 and 3 days), a card with pupae parasitoid was placed inside transparent plastic petri dish ( 6 cm × 4 cm ) with treated (using the insecticide) or with control (using water) olive leaf samples. A 2 × 2 cm aeration hole (4 cm2) covered with fine cotton cloth was made on the petri dish to assure continued ventilation in the area. In total, 30 cards for each Trichogramma specie (Each card represented a single independent replicate): 15 cards (n = 15 per treatment) and 15 cards (n = 15 per control) were conducted at 25˚C ± 2˚C, 60% ± 5% RH, and 14 L: 10 D.
2.4. Analyses of Pesticide Residues
2.4.1. Olive Leaf Sampling
Other samples of olive leaves were collected from same young olive trees maintained in greenhouse at each time after the treatment: starting from the day of spray (one hour after pesticide application) and at different time intervals (3, 10, 17, 24 and 31 days). Leaf samples were collected randomly from each tree and cut in fine pieces (about 5 mm diameter), placed immediately in a cool-box held at 4˚C - 8˚C after collection and transferred to refrigerator for analysis within 24 - 36 hours.
2.4.2. Extraction
Fifty gram samples collected at each time intervals were blended in 100 ml of acetonitrile?water (80/20; v/v) for 2 min, shaken for approximately 30 min and filtered through a buchner funnel than washed with acetonitrile- water mixture (8:2, v/v). Extract was purified using silica SPE (Solid Phase Extraction) cartridge. The acetonitrile solution was eluted, collected and immediately evaporated to dryness in a rotary vacuum evaporator. The residue was reconstituted in 1 mL of methanol: acetonitrile: 2% ammonium acetate solution (1:1:1, v/v) for final HPLC analysis (HPLC, a Beckman model 322) equipped with UV detector (250 nm). The column used was C18 ( 250 mm × 4.6 mm i.d). The mobile phase was acetonitrile: methanol: 2% ammonium acetate (21:21:8) at a flow rate of 2.0 mL∙min−1. A 5 μL aliquot of each sample was injected each time for residue analysis. The representative retention times of spinosyn A and D were 9.5 min and 11.0 min, respectively.
2.5. Data Analyses
Parasitism viability (number of emerged adults from parasitized pupae) and adult emergence time (developmental time from pupa to adult emergence) were evaluated.
The effect of the insecticides was established by comparing the controls with the treatments using the following formula:
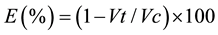
where E is the insecticide effect in parasitism viability (number of emerged adults from parasitized pupae) compared to the control; Vt is parasitism viability observed for each insecticide treatment and Vc is the parasitism viability observed in the control ones. The value E calculated for the insecticide treatments were classified according to the International Organization of Biological Control (IOBC): class 1―harmless (E < 30%), class 2― slightly harmful (30 % < E < 79%), class 3―moderately harmful (80 % < E < 99%) and class 4―harmful (E > 99%) [25] . Proportional data were normalized through arcsine transformation; all the other data were normalized through square root transformation.
The tests were performed using the Statistical Package for the Social Sciences (SPSS) for Windows (version 17.0). A complete randomized factorial design was used, and data were submitted to analysis of variance (ANOVA, p < 0.05). Significant mean values associated with parasitism viability were compared by Dunnett’ test.
Tukey test for multiple comparisons was performed on the various groups to determine whether differences associated with adult emergence time existed between treatments and exposure periods.
In the text and figures, mean ± SD (standard deviation) are used as descriptive statistics for distributed data
3. Results
3.1. Insecticide Effect on Trichogramma Species
Results from the experiment are presented in Table 1. The dominant class was 2 (30% < E < 79%) for all treatments and parasitoid species combinations. However, the classification changed from class 2 to class 1 (E < 30%) or to class 3 (80% < E < 99%). In fact, result from treatment using olive leaf samples on day 31 after deltamethrin spraying has show a slightly harmful effect on parasitism viability, except the case for T. oleae where the insecticide was harmless (E% = 23.6 ± 8.3). However, deltamethrin was moderately harmful to T. bourarachae (E% = 80.5 ± 2.2) on day three. Spinosad was harmless to T. oleae and T. cacoeciae at 10 days (E% = 18.8 ± 9.0). However, it was slightly harmful to T. bourarachae even on day 31 (E% = 32.3 ± 4.8).
3.2. Comparison between Insecticide Effects on Trichogramma Species Parasitism Viability
Table 2 presents the pair multiple comparisons among the treatments associated with parasitism viability for the different assessment periods.
The spinosad treatment significantly reduced parasitism viability of all three Trichogramma species for all assessment periods. Difference in the mean values associated with parasitism viability between spinosad treatment and control were 30.7 for T. bourarachae, 22.0 for T. oleae and 26.0 for T. cacoeciae (P < 0.05). In addition, parasitism viability of all the three Trichogramma species was less affected by spinosad than by deltamethrin (P < 0.05).
3.3. Adult Emergence Time of Trichogramma Species at the Different Exposure Periods
Adult emergence time (Pupa-adult development time) of Trichogramma reared on E. kuehniella eggs differed among the three species in their response to insecticide residues at the different exposure periods. Periods of the experiment in the control group do not affect adult emergence time of: T. oleae (Table 3, Tukey test, F = 0.62; df = 4; p = 0.854), T. bourarachae (F = 0.5; df = 4; p = 0.736) and T. cacoeciae (F = 0.71; df = 4; p = 0.780). Wasp development from pupa to adult emergence at 25˚C normally requires about 6 days. However, pupa exposed to the insecticide residues required about one to two days longer to complete development. Adult emergence time of T. oleae was significantly long when pupa were exposed to deltamethrin residues at 3 days after the treatment. More significant decrease in adult emergence time was observed at 10 and 17 days then at 24 and 31 days (Table 3, Tukey test, F = 91, 45; df = 4; p < 0.05). However, adult emergence time from T. oleae pupa exposed to spinosad residues was significantly decreased at 24 days after the treatment (Table 3, Tukey test, F = 31.67; df = 4; p < 0.05).
Adult emergence time from T. cacoeciae pupa decreased when exposure period to deltamethrin and to spinosad residues occurred, respectively, at 31 days (Table 3, Tukey test, F=42.27; df = 4; p < 0.05) and at 10 days (Table 3, Tukey test, F = 29.83; df = 4; p < 0.05) after the treatment.
In contrast, adult emergence time from T. bourarachae pupa exposed to deltamethrin or to spinosad residues was similar in all the periods of exposure (Table 3, Tukey test, F = 0.72; df = 4; p = 0.624 and F = 0.52; df = 4; p = 0.736).
![]()
Table 1. Insecticide effect (E) on parasitism viability reduction associated with T. oleae, T. cacoeciae and T. bourarachae after exposure of parasitoid pupae to residues on leaves at different times (days).
1E(%) = (1 − Vt/Vc) × 100, where E is the effect of the insesticide on parasitism viability reduction compared to the control, Vt is the parasitism viability for the treatments (using insecticide) and Vc is the parasitism viability for the control (using water). 2Class: 1. harmless (E < 30%); 2. slightly harmful (30% < E < 79%); 3. moderately harmful (80% < E < 99%); 4. harmful (E > 99%).
![]()
Table 2. Comparison between the deltamethrin and spinosad treatments based on parasitism viability of Trichogramma species.
Mvd. Mean value differences *Significant difference (p < 0.05, Dunnett’s test).
![]()
Table 3. Effect of deltamethrin and spinosad treatments on the pupa-adult development time (adult emergence time) of Trichogramma species after the different exposure periods.
a. Numbers in column followed by different letters are significantly different (Tukey test: p < 0.05).
3.4. Parasitism Viability of Trichogramma Species Based on the Treatment Periods
Percentage of reduction in parasitism viability of the three Trichogramma species differed between treatment and exposure time (days after spraying leaves). Figures 1-3 present percentages of parasitism viability reduction for each Trichogramma specie and corresponding exposure times. It appears that exposure time after insecticide spray had a significant effect on Trichogramma parasitism viability.
![]()
Figure 1. Reduction in parasitism viability (%) of T. oleae at days after spraying leaves.
![]()
Figure 2. Reduction in parasitism viability (%) of T. cacoeciae at days after spraying leaves.
![]()
Figure 3. Reduction in parasitism viability (%) of T. bourarachae at days after spraying leaves.
However, the effect seemed to differ not only from the spinosad to deltamethrin treatments but also from specie to specie. The deltamethrin treatment reduced parasitism viability of T. oleae at 50% even 17 days after parasitoid pupae exposure to residues on leaves. In fact, T. oleae displayed a parasitism viability of 80.3% in control assessment, reduced to 40.15% at 17 days after the treatment. Percentage of reduction in parasitism viability of T. oleae decreases to 36% on day 24% and to 13% on day 31 (Figure 1).
Spinosad exhibited low toxicity 10 days after exposure. For T. oleae, parasitism viability was similar to the control at 78.9% on day 24 and percentage of reduction in parasitism viability was 4.51% (Figure 1). For T. cacoeciae, deltamethrin was persisted at 31 days after the treatment showing a negative effect on parasitism viability, reduced to 44% (Figure 2). Also, spinosad exhibited a negative effect on parasitism viability at 17 days after the treatment but 24 and 31 days after exposure to the treatments, reduction in parasitism viability rates decreased to 21% and 7.05% respectively (Figure 2).
For T. bourarachae, reductions in parasitism viability curves for exposure time have the same shape for both insecticides. Indeed, reduction in parasitism viability gradually decreases with increasing time after insecticide spraying (Figure 3). Deltamethrin and spinosad exhibited high reduction in parasitism viability of T. bourarachae at 3 days after treatment (72.13% and 66.70% respectively) but parasitism viability declined much more in than was the case for T. oleae or T. cacoeciae. At 31 days after parasitoid pupae exposure to residues on leaves, parasitism viability of T. bourarachae was reduced for the deltamethrin treatment at 35.5% and for the spinosad treatment at 25.5% (Figure 3).
4. Discussion
Parasitoids in the genus Trichogramma are reported to be excellent indicator species associated with the side effects of pesticides [25] . It was found that insecticides affected Trichogramma emergence from host eggs when exposed at different preimaginal stages of development (larval, prepupal, or pupal) [7] . The present study was designed to evaluate three Trichogramma species: T. oleae, T. cacoeciae and T. bourarachae for their susceptibility to deltamethrin and spinosad when exposed at pupal stage of development. These parasitoids were candidate to be used in pest management programmes against the second generation of P. oleae, as it is especially difficult to be managed with insecticides in most olive areas in Tunisia.
The insecticide concentrations tested were equivalent to those recommended for field application. Side effect evaluation is carried out by measuring parasitism viability rates and adult emergence time of Trichogramma species exposed to residues on olive foliage at different time intervals. These measures are important in order to investigate compatibility of Trichogramma species with insecticide residues after exposure under semi-field conditions. Most laboratory and field studies have shown that Trichogramma wasps are highly susceptible to broad-spectrum insecticides [26] . Consequently, use of insecticides and Trichogramma has historically been considered incompatible. The insecticides from the group of Piretroids were commonly described as harmful to beneficial arthropods [27] . According to the IOBC standard methods, deltamethrin is classified as harmful for adults and as slightly harmful for pupae of Trichogramma [28] . Similarly, this study demonstrate that exposure to deltamethrin resulted in parasitism viability reduction of the three Trichogramma species and showed slightly harmful effect for the parasitoid pupae of T. oleae and T. cacoeciae (30% < E < 79%). However, T. bourarachae exhibited moderately harmful effect 3 days after pupae exposure to deltamethrin residues on leaves (80% < E < 99%). Additionally, data show that residues from the deltamethrin treatments were remaining after 24 days. This result indicates that persistence ranged widely, from three to 31 days.
Spinosad, which is obtained from a naturally occurring soil organism, is not considered without risks on beneficials such as predatory insects [29] . However, although spinosad was slightly harmful at the outset of this study (30% < E < 79%), reduction in parasitism viability decreased after a period of exposure to the treatment. This result is similar to this reported by [12] , showing that spinosad degrade quickly following application in the field with residues no longer toxic by 7 to 10 days post-application. However, it appears from our results that susceptibility to spinosad differed from specie to another even after a period of exposure to the treatment.
Overall, the present experiment demonstrated that spinosad was less harmful, based on effect on parasitism viability, than deltamethrin to the three Trichogramma species. At the label rates, spinosad was harmless (E < 30%) to T. oleae and T. cacoeciae and slightly harmful (30% < E < 79%) to T. bourarachae after exposure at 31 days. Spinosad appears to be more compatible with the three Trichogramma species than deltamethrin which exhibited moderately harmful effects to T. bourarachae (80% < E < 99%).
Differences regarding susceptibility to the two insecticides show that T. cacoeciae was more susceptible to spinosad than T. oleae. Also, T. bourarachae was more susceptible to the insecticides than T. oleae and T. cacoeciae with a decrease in parasitism viability when compared to the control. Besides longevity and fecundity that are key attribute for the quality of commercially available Trichogramma, the result of this study is important in biocontrol programs where the choice of less susceptible specie to insecticide can be critical.
Although some studies showed that pre-imaginal stages developing within host eggs appear to be protected from many insecticides, [26] reported that spinosad and other piretroids adversely affected Trichogramma emergence from host eggs when exposed at different pre-imaginal stages of development (larval, pre-pupal, or pupal). In addition, our results indicate that Trichogramma pupa is susceptible to residues of spinosad as well deltamethrin weathered on olive leaves for up to 3 at 31 days.
Adult emergence time (Pupa-adult development time) appeared to be increased for 1 to 2 days when Trichogramma pupa were exposed to insecticide residues at the shortest period after treatment, whereas, exposure at longer periods after the treatment seemed to eliminate this effect, especially, for T. oleae and T. cacoeciae. Thus, the increased length of the adult emergence time is likely related to the susceptibility of Trichogramma species in their response to insecticide residues at the different exposure periods.
Residue analysis provides a measure of the nature and level of any chemical contamination within the environment and of its persistence. However, it is often difficult to correlate pesticide residues in the environment with effects on fauna and/or ecological processes. Furthermore, when using insecticides, the relationship between the toxicant and the unit of live is greater for smaller organisms [30] . Thus, we were interested to show whether the insect parasitoid has been exposed to direct toxic effect of chemicals and to identify its potential use for future biological control programmes in the environment.
For the pesticide risk assessment, the exposure characterization describes the actual contact of pesticides and its residue with the parasitoid and the objective was to describe exposure in terms of live, susceptibility and parasitism viability of Trichogramma species. This study show that exposure of Trichogramma species to spinosad is probably only of concern for 3 - 15 days after spraying, However, exposure to deltamethrin residues is likely to be persistent effect on the parasitoid.
Even experiments were carried out under laboratory conditions where the parasitoid was probably submitted to the highest insecticide pressure, Trichogramma release period might be expected, avoiding harmful insecticide residue, to make a better contribution to P. oleae second generation control. More generally this can suggests that an insecticide-free environment needs to be considered to ensure maximum parasitism viability of Trichogramma in the field. Thus, under field conditions, adults’ emergency from parasitized pupa the time of release, would not be affected by the residual activity of the insecticides if Trichogramma emergence occurred 24 days after treatment (case of T. oleae and T. cacoeciae) and 31 days after treatment (case of T. bourarachae).
Nevertheless, this prediction requires validation by field studies for example under particular dry conditions that may result in greater persistence of residues [14] .
Releasing native species is important since these species have the advantage that they are already adapted to the local environmental conditions of olive tree. However, the susceptibility of a Trichogramma parasitoid to the insecticide may be influenced by the ecological attributes of the introduced species [31] . This is, in fact, one of the reasons that make the introduction of Trichogramma species to the field conditions an interesting topic of study in the broader area of insecticide effects. This laboratory study must be validated with a field one studying the interactions between biotic performance of Trichogramma specie with the weather, foliage, selective forms of application and dosages of insecticides, to obtain an adequate relationship between effectiveness of treatment with the parasitoid fecundity. Accordingly, further studies are currently under way to gain better insights on Trichogramma species effectiveness for biological control programs.
Acknowledgements
The authors wish to express their gratitude to Dr. Annette Herz (BBA, Germany) for her Technical Cooperation within the International project INCODEV (TRIPHELIO): Sustainable Control of Lepidopterous Pests in Olive Groves-Integration of Egg Parasitoids and Pheromones. They would also like to thank all technicians in the Unit of Mass production and maintain of Trichogramma in Olive Tree Institute of Sfax, Tunisia.
NOTES
*Corresponding author.