Characterization of Dye Sensitized Cells Using Natural Dye from Oil Bean Leaf (Pentaclethra macrophylla): The Effect of Dye pH on the Photoelectric Parameters ()
1. Introduction
The concept of dye sensitization of wide band gap mesoporous metal oxide semiconductors began by the work of [1] [2] in 1960 with collaboration of their co-workers; they used several dyes as photo sensitizers and zinc oxide (ZnO) as semiconductor. ZnO is a compound of group II, VI elements. Its unique applications and semiconductor properties are drawing attention from researchers because of its diverse uses in everyday life. ZnO has high electron mobility [3] , strong-luminescence at room temperature and good transparency. Efficiencies observed were very low for many years [4] . In 1991, progress was made when [5] [6] obtained efficiency higher than 7% from using TiO2 with a Ruthenium based sensitizer as semiconductor material [6] . DSSC research increased its impact worldwide [7] after Ruthenium-based dye was used and a higher efficiency; 11% was recorded in a cell made with TiO2 nanoparticles in a photoelectrochemical cell (PEC) device. Nanocrystalline TiO2 existed in five different forms; TiO2 (anatase) was used because it was abundant, safe and very stable. TiO2 has a wide band gap (Egrutile = 3.0 eV; Eganatase = 3.3 eV). DSSC technology based on PEC holds out the promise of cheaper solar cells significantly less than customary silicon cells. The PEC is an electrolyte-based semiconductor device that generates photovoltage when illumined and it is photocurrent when external circuit is closed. The short circuit current is a linear function of intensity of illumination, so the device does not need external bias. Investigations revealed cheaper manufacturing costs and faster process of producing DSSC modules.
Transport and light absorption in DSSC are separated unlike in silicon solar cells where the sensitizer harvesting the light is attached to a wide band semiconductor surface [6] . At the boundary, charge separation through light induced electron transmission from the into the semi-conductor conduction band of the dye to the charge collector [8] -[12] . At present, ruthenium polypyridyl complex manufactured from heavy transition metal compound is most commonly used because of its good absorption, long excitation lifespan, highly efficient metal-to-ligand charge transfer (MLCT) [13] and strong charge-transfer (CT) absorption in the visible spectrum of light. Complexes of Ruthenium are however difficult to prepare as well as expensive; this restricts their applications on a large-scale in solar cells, creating a need for cheaper alternatives like organic cells [14] . DSSC’s with efficiencies of up to 9% using organic cells that have properties like Ruthenium have been investigated [15] -[17] . Organic dyes used as dye sensitizers in DSSC’s are from fruits, plants, leaves, pulp and other natural sources [18] -[20] . Most leaves, flowers and fruits contain different pigments that are used in DSSC’s. Green leaves are usually rich in chlorophyll; its application as dye sensitizers has been researched by many scientists [9] [17] . Anthocyanins are responsible for the colour of plants and fruits, the red colour of petals, purple-red color of autumn leaves and the rich olive green of the oil bean leaf. In this paper, flavonoid and anthocyanin extracts of the hexane faction of P. macrophylla were used as dye sensitizer at different pH in DSSC’s.
P. macrophylla Benth (Oil Bean) tree is a member of the “Fabaceae” family [21] , Leguminosae-Mimosoideae sub family, of genus Pentaclethra. It has some local names: African oil bean, Atta bean, Congo acacia, Ngazi (English), Owala, Mubala, Owala oil tree, arbre à acacia cu Congo (French), and Sucupira, Marróne (Poland). P. macrophylla provides food for West Africans, all through the rain forest belt. Its seeds are roasted or boiled; when fermented, the seed is served as a meaty flavoured snack, “Ugba”. The dry empty pod is used as a source of fuel. P. maloba also belongs to the same genus and family, but it is found in temperate regions of the world. P. macrophylla seed oil can be extracted for fuel, used in cooking, food preservation, spices and condiment. The leaves, when they fall, improve soil fertility. P. macrophylla wood “mubala” or “ovala” is suitable for making charcoal and fuel. Ash from its pods, wood or tannins is used as a mordant for dyeing in Ghana. Household utensils and decorations are carved from its wood in Nigeria and Ghana. Its seeds are decorative and are used in beads, rosaries and necklaces. It is nitrogen-fixing plant and so enriches the soil nutrient content [22] . Two years after cultivation, P. macrophylla develops a measure of resistance to fire and when lopped; it sprouts easily. Species grow relatively fast and attain a height of about 1.5 metres in first year after on good soil and favourable weather conditions. The wood is strong and hard, but difficult to work.
2. Experimental Details
2.1. Extraction of P. macrophylla Leaf Dye
Leaves of P. macrophylla were dried in air until it crumbled dry crisply. It was milled to coarse particles, and spread out to dry to prevent it from becoming moldy. A phytochemical screening was done on the leaf sample to identify the active chromophores. P. macrophylla was soaked in methanol for eleven days to extract the dye. This dyesol (dye solution) mixture was separated using plastic funnels and cotton wool. The filtrate was collected in large sample bottles and covered. This was then poured periodically into a rotary evaporator, to separate the dye from the mother liquor for future use in the laboratory.
2.2. Batch Separation of Dye
A separating funnel was used to purify the feedstock. The crude extract (dye) was mixed with 40 ml Hexane and 20ml Ethyl Acetate [23] [24] . This mixture was thoroughly mixed and poured into the flask of the separating funnel to obtain the different sections. The four portions obtained were:
a) The crude;
b) Aqueous;
c) Hexane;
d) Ethyl Acetate factions.
The dry crude and hexane portions had the largest percentage; the aqueous and ethyl acetate factions were very small.
Test for Flavonoids
The solution of the extract was tested with the following reagents:
Shinoda Test: 3 ml of extract was added to a small quantity of magnesium turnings, and then a few drops of concentrated hydrochloric acid were run down the side of the test tube. At the upper phase, the appearance of three colour bands; orange, pink, reddish indicated the presence of flavonoid (Table 1).
Test for Anthraquinones
Free Anthraquinones: About 0.5 g of the extract was shaken with 5 ml of chloroform for ten minutes and filtered. The filtrate was shaken with 5 ml of ammonia solution. Presence of a pink colour in the ammoniacal phase indicated the presence of free anthraquinones (Table 1).
Combined Anthraquinones: 1 g of powdered extract was boiled with 5 ml of 10% hydrochloric acid for five minutes and filtered while hot. The cool filtrate was partitioned against equal volume of chloroform (two volumes), avoiding vigorous shaking. A clean pipette was then used to transfer the chloroform layer to a test tube taking care not to include the aqueous layer. An equal volume of 10% ammonia was added to the chloroform extract. A pink, red or violet colour in the aqueous layer indicates the presence of combined anthraquinones (Table 1).
2.3. Preparation of DSSC
Commercial TiO2 powder (Assay 98% min) variety was used. The TiO2 film was prepared by smoothening 12 g TiO2 in 20 ml of concentrated nitric acid for about 1hour and sintered in the oven to properly fuse the TiO2 molecules and to enhance its absorption performance. The whitish paste was spread on Fluorine doped tin oxide (FTO) conducting glass with dimensions: 50 mm × 50 mm × 22 mm and surface resistivity 7 Ω/m2 (735140- 5EA. ALDRICH). Screen printing method and rigid squeegee was used to deposit TiO2 paste on the FTO. TiO2 nanoparticles thus produced had an average particle size of 20 nm. The DSSC’s active area was 6.25 mm2 (≈ 2.5 mm × 2.5 mm).
The sintered TiO2 was immersed in P. macrophylla dye, allowing the dye to be adsorbed on TiO2 nanoparticles. Methanol was used to clean off any dye trickle on the FTO. After cleaning the DSSC’s, the photoelectrode was assembled. Soot was coated on the conducting side then, each unit of DSSC was held firmly with crocodile clips; Allowing a little space at the edges to enable injecting the dye sensitizers or electrolyte.
2.4. Current Voltage Characterization
The performance of the DSSC under AM1 atmosphere was measured using a multimeter (Voltcraft; M-3850 series). Results obtained was plotted to obtain an I-V curve, from which the open circuit voltage Voc (V), short- circuit current density Jsc (mA/cm2), fill factor (FF) and conversion efficiency η% was determined.
3. Results and Discussion
Figure 1 shows the Fourier Transform Infrared spectroscopy (FTIR) spectrograph of P. macrophylla leaf dye
![]()
Table 1. Phytochemical analysis of P. macrophylla.
extract used as dye sensitizer on sintered TiO2 nanoparticle. It reports [25] functional groups and types of bonds present in an organic compound from a list of absorption frequency and peaks shown by its wave number.
FTIR is very useful in the analysis of metal complexes and fluoromanganates contained in organic com- pounds. A detailed analysis of compounds present in P. macrophylla is shown in Table 2.
An absorption peak (A.P) 426.28 cm−1 indicates any iodoalkane-type bond (C-X), its appearance is medium to strong. 673.18 and 731.05 (cm−1) reveals any chloroalkane (C-X) whose presence is medium to strong. A.P of 835 cm−1 tells of strong para-disubstituted aromatic benzene. 1041.60, 1078.24 and 1163.11 (cm−1) specifies (C-X) ordinary and trifluromethyl fluoroalkanes respectively having two strong broad bands. A.P 1240.27 cm−1 conveys presence of aromatic ethers (C-O). Alkyl (C-H) bonds are expressed by 1377.22 cm−1, 2852.81 cm−1 and 2926.11 cm−1; methyl-type of weak, medium to strong appearance respectively. Three or four Aromatic C=C bonds of weak to strong appearance is indicated by 1458.23 cm−1 A.P. Strong aliphatic nitro compounds (N-O) is contained in 1535.30 cm−1. A.P of 1618.33 cm−1 and 1664.62 cm−1 reveals C=N bond with similar conjugation effects to C=O. 1712.85 cm−1 and 1735.99 cm−1 shows carboxylic acids (C=O) derivatives of saturated type, esters and lactones (influenced by conjugation and ring size) respectively. A.P 2727.44 cm−1 communicates any medium sized aldehyde bond (C-H). Multiple broad peaks of ammonium ions is shown by 2956.97 cm−1.
![]()
Table 2. FTIR analysis of compounds in P. macrophylla’s leaf extract.
FTIR analysis of P. macrophylla’s constituents.
Figure 3 shows an absorption range of P. macrophylla dye from 702 - 774 nm with an absorption peak at 670 nm and absorbance of 0.053 au without any electrolyte sensitizer. The absorption spectra reveal the transition between the ground state and excited state of the P. macrophylla dye and the absorption width of solar energy wavelength.
Figures 4-6 show the absorption spectra of P. macrophylla with potassium iodide (KI), potassium bromide (KBr) and mercury chloride (HgCl2) electrolytes respectively compared with P. macrophylla dye having no sensitizer for dye. The KI sensitized DSSC (Figure 4) had an absorption in the frequency range 408 - 1086 nm with an absorption peak at 408 nm and absorbance of 0.592 au. A series of interesting events unfold: between 300 nm to 400 nm a π to π* transition occur at 0.592 au. Recombination occurs between 408 nm and 470 nm. Another π to π* transition occur between 470 nm and 520 nm. Consequent loss of energy follows recombination at 570 nm to 620 nm. A third π to π* jump occurs at 700 nm to 750 nm, 750 nm to 800 nm witnesses a loss of energy due to recombination. Finally, it attains stability after 800 nm [26] . The chlorophyll dye extract with the KBr and Hg Cl2 as sensitizer also had absorption peaks at 408 nm but at 0.851 and 0.634 absorbance respectively. Chlorophyll has its highest absorbance within the blue and red bands of the absorption spectra. Figure 5 has similar features as Figure 4. Its first transition occurs at 320 nm to 400 nm at 0.851 au. However, recombination is longer 408 nm to 480 nm. At 500 nm to 504 nm wavelength, a small jump π to π*occurs at 0.151 au. A higher jump π to π* happens at 669 nm at 0.360 au before a consequent recombination [27] and resulting stable state. HgCl2 reveals an initial boost as energy level of P. macrophylla starts at a good peak of 0.628 au absorbance, a drop due to recombination of holes with electrons occur from 300 nm to 330 nm. A π to π* transition occurs at 330 nm to 408 nm at 0.634 au. Recombination and resulting energy drop follow before a final stable state. Figures 4-6 show absorption peak of 408 nm which indicates that the dye has same colour composition but different absorbances due to differences in the compositions of the electrolytes [26] [27] . The weak absorption of the P. macrophylla in the red region is a disadvantage, this affects the DSSC efficiency. The optimum use of dye sensitizers, solution of electrolyte and surface morphology of metal oxide will improve on DSSC performance.
Operational parameters like open circuit voltage Voc, short circuit current Isc, current and voltage at maximum power point of a solar cell are obtained from the current-voltage (J-V) curves of the solar cell. Figure 7 shows the J-V characteristics of the fabricated DSSC using P. macrophylla leaf extract and KI, KBr and HgCl2.
Table 3 shows data obtained from determining the photoelectric conversion efficiency of DSSC’s P. macrophylla.
Conversion efficiency of DSSCs sensitized with P. macrophylla dye and KI electrolyte is 0.21%, with Voc of 0.032 V, Jsc of 0.2 mA/cm2 and fill factor (FF) of 0.480. Conversion efficiency for DSSC sensitized with P. macrophylla and KBr is 0.43%, Voc is 0.045 V, Jsc is 0.16 mA/cm2 and FF of 0.28. The conversion efficiency for DSSC sensitized with HgCl2 is 0.36% , with Voc of 0.043 V, Jsc of 0.12 mA/cm2 and FF of 0.23. The equation used for determining the efficiency is:
![]()
Figure 2. Fourier transform ınfrared spectrograph of hexane faction of P. macrophylla dye.
![]()
Figure 3. UV/Vis of P. macrophylla without dye sensitizer.
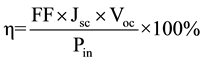
Pin is power intensity of incident radiation [28] .
The result in Table 3 shows the DSSC sensitized with HgCl2 had the highest efficiency while that sensitized with KI had the least photoelectric conversion efficiency. The Voc of P. macrophylla dye was less than ruthenium dye this could be due to the functional groups present in the organic dye; typically O and OH ligands which do not have-COOH ligand that combine readily with TiO2’s hydroxyl particles forming esters which cause an increased rate of electron-transport [29] . These values are close to those for DSSCs of frozen Blackberries [14] where Voc was reported as 0.33 V and 0.4 V and 0.59 V as Voc for Jabticaba’s skin and dye extracts of
![]()
Figure 4. UV/Vis spectrograph of P. macrophylla with KI dye sensitizer.
![]()
Figure 5. UV/Vis spectrograph of P. macrophylla with KBr dye sensitizer.
![]()
Figure 6. UV/Vis spectrograph of P. macrophylla with HgCl2 dye sensitizer.
![]()
Figure 7. J-V characteristics of DSSCs with KI, KBr and HgCl2 dye sensitizers with P. Macrophylla extracts dye.
![]()
Table 3. Characteristics of P. macrophylla dye-sensitized solar cells.
Photoelectric parameters of P. macrophylla DSSCS.
blueberries respectively [30] .
4. Conclusion
Dye sensitized solar cells were prepared from anthocyanin extracts of P. macrophylla and a mixture of dye sensitizers; KI, KBr and HgCl2. The photoelectric conversion efficiency with HgCl2 attained 0.43%, while the conversion efficiency with KI as dye sensitizer was only 0.21%. The DSSC with HgCl2 in this study gives a higher incident photon-to-electron conversion. Sustaining the visible high peaks, less recombination of holes with electrons ejected from electrolytes is subject to future research. Dye substrates enclosed in substances like plasma gel and centrifugal paste are a quest for prospective research, as the effect of dye sensitizers on DSSCs photoelectric conversion efficiency continues to be further researched.
Acknowledgements
The authors appreciate the staff of the Chemical Science Department, Redeemer’s University, Ede and staff in the Instrumentation laboratory of the Covenant University, Ota for the use of their research facilities.