A Flexible Quasi-Solid-State Electrochromic Device with Polymeric Electrolyte and WO3/NiO Complementary System ()
1. Introduction
Electrochromism is the phenomenon related to the optical changes during a reversible electrochemical process of some materials. This phenomenon was first reported by Debin 1969 [1]. Electrochromic materials have been attracting much interest for their potential applications including electrochromic windows [2] [3], rear-view mirrors [4], electrochromic displays [5], and electronic paper or electrochromic painting [6] [7]. Inorganic electrochromic materials, such as Prussian blue [8] and transition metaloxides (e.g., W, Ni, Ir, V, Ti, Co and Mo), have been studied for a long time [9]. Among the numerous electrochromic materials, tungsten oxide (WO3) is one of the most important materials for its high transmittance attenuation, good coloration efficiency and low response time [10]. WO3 thin film can be prepared by several methods, such as anodicoxidation [11], spraypyrolysis [12], sol-gel synthesis [13], electrodeposition [14] and sputtering [15]. Compared to the cathodic coloration material of WO3, nickel oxide (NiO) is a typical anodiccoloration material and has drawn much interest for the last decades. These two electrochromic materials can be fully transparent and assembled to a complementary electrochromic device (ECD) [16].
The electrolyte is one of the important parts of the ECD assembly. Similar to the case of lithium battery, liquidelectrolyte has the leakage and safety problem. Several methods have been demonstrated to reduce the electrolyte leakage by using ionic liquid [17], polymer gel [18], and solid-state or quasi-solid-state materials [19]. Polymeric electrolytes have become the most promising electrolyte materials for practical application due to the low cost and good stability. In this study, WO3 and NiO thin-fim electrodes, which are prepared by using sputtering, are chosen to fabricate a complementary ECD. A polymeric composite, which is composed of a UV- cured ethoxylated trimethylolpropane triacrylate (ETPTA), propylene carbonate (PC) and ferrocene (Fc), is used as the polymeric electrolyte in the quasi-solid-state ECD. The influence of the polymeric electrolyte on the ECD’s optical performance is discussed in this paper.
2. Experimental
2.1. Preparation of the WO3 and NiO Thin-Film Electrode
The preparation processes of the WO3 and NiO thin-film electrodes are described as follows: First, the optically transparent Sn-doped indium oxide (In2O3:Sn, or ITO) was prepared on glass or PET substrates by pulsed dc magnetron reactive sputtering with our homemade instrument. The ITO electrodes were cleaned ultrasonically in DIW for 1 min. WO3 and NiO thin film were deposited onto the ITO electrodes by sputtering for 2 and 1 hr, respectively. The thickness of ITO, WO3 and NiO thin films were 110, 70, and 60 nm, which were measured by a profilometer. The electroactive area of the WO3 and NiO thin-film electrodes were 2.0 × 2.0 cm2.Copper tape was applied to one side of the ITO as the bus bar. The WO3 and NiO thin-film electrodes were stored in air at room temperature before device assembly. To record the spectroelectrochemical properties of the thin-film electrodes, apotentiostat/galvanostat (CHI 900B, CHI, USA) and aspectrophotometer (SolidSpec-3700, Shimadzu, Japan) were used.
2.2. Assembly of the WO3/Polymeric Electrolyte/NiO ECD
Before the device assembly, both the WO3 and NiO thin-film electrodes were pretreated with the activation process as follows: The WO3 thin-film electrode was cycled several times in anelectrolyte using the cyclicvoltammetric (CV) method in a two-electrode electrochemical system, to ensure the function of the lithium ion insertion and extraction. The counter electrode was a platinum electrode. The electrolyte was composed of 0.1 M LiClO4 and PC. For the NiO thin-film electrode, a solution containing 1 M NaOH and deionized water was used as the electrolyte. The NiO thin-film electrode was cycled back and forth until a stable current signal was observed. The detail of the ECD assembly is described as follows: a 0.1 ml monomer solution of the polymeric electrolyte was dropped onto the WO3 thin-film electrode and then covering it with the NiO thin-film electrode. The UV-cureable monomer solution contained 15 wt% of ETPTA and 85 wt% of PC with 0.1 mM Fc and 1 M LiClO4. The sandwich-like ECD was exposed under UV-irradiation for 60 s and then a quasi-solid-state ECD was formed. The ECD was allowed to cool down to the room temperature, and then was sealed with a UV- curable blocking adhesive around its four edges.
3. Results and Discussion
3.1. Cyclicvoltammetric (CV) Analysis of WO3 and NiO Thin-Film Electrodes
Figure 1 shows the CV analyses and the UV-v is transmittance spectra of the WO3 thin-film electrodes during the activation processes. In Figure 1(a), a typical asymmetrical CV curve of the redox reaction of WO3 can be observed. The redox reaction of the WO3 can be represented by the following equations [16]:
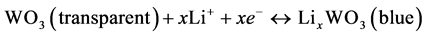 (1)
(1)
To ensure the smooth ion insertion and extraction at the WO3 thin film, it was scanned between −3.3 and 2 V
![]()
![]() (a) (b)
(a) (b)
Figure 1. (a) The CVs of WO3 thin-film electrode in 0.1 M LiClO4 PC solution; (b) The transmittance spectra of the WO3 thin film at different applied potentials.
for 100 cycles in a two-electrode electrochemical system. When the potential was more negative than −0.5 V, the Li ion inserted into the WO3 thin film and color changed to light blue slightly. The color of the film became deep blue at the applied potential of −3.3 V and then changed to transparent during a reverse scan. During the 100-cycle pretreatment, the current density of the electrode became stable, and it indicated that the equilibrium of the ion insertion and extraction was achieved. The transmittance difference between the bleached state and the colored state of the WO3 thin film was performed in Figure 1(b). The transmittances at 550 nm of the fully bleached and colored state of the WO3 thin film were 76% (at 1.5 V) and 20% (at −3.5 V), respectively.
Figure 2(a) shows the CV curves of NiO thin-film electrode during the activation processes. The electrode was cycled between −1.5 and 1.5 V in a two-electrode electrochemical system. An obvious increase of the reduction current can be observed in the first ten cycles of the CVs, which can be attributed to the redox reaction of NiO thin film, as represented by Equation (2) and (3) [16]:
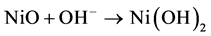 (2)
(2)
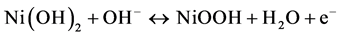 (3)
(3)
During the continuous scanning of the NiO thin film in the alkaline solution, NiO transformed to Ni(OH)2 gradually and became more electrochemically active. Thus, the reduction peak at −0.75 V became larger with the increasing cycle number. The color of the NiO thin film changed reversibly from dark brown (at 1.5 V) to transparent (at −1.5 V). From the transmittance spectra shown in Figure 2(b), a transmittance peak of NiO at 900 nm could be observed. The transmittances at 900 nm of the fully bleached and colored state of the NiO thin film were 75% (at −1.5 V) and 45% (at 1.5 V). The largest transmittance difference was 38% at 600 nm.
3.2. Performance of the WO3/Polymeric Electrolyte/NiO ECD
Figure 3 shows the schematic diagram of the quasi-solid-state ECD, and the inset is the polymeric electrolyte. The polymeric electrolyte was composed of EPTPA, LiClO4, ferrocene and PC. After exposed to UV irradiation, the freestanding electrolyte film was obtained. The color of the polymeric electrolyte is yellowish because ferrocene was added into the electrolyte. This quasi-solid-state polymeric electrolyte made it more convenient to assemble the ECD. The CV responses of the WO3/polymeric electrolyte/NiO ECD are shown in Figure 4. The ECD was scanned between −2.5 and 1.5 V at a scan rate of 20 mV・s−1. The figure shows two sets of redox reactions, which can be distinguished by comparing to the CVs of WO3 and NiO thin films. The first set of the redox reaction at the voltage between −2.5 and 0 V is contributed by the WO3. When the applied voltage was more negative than −2.0 V, the color of the device changed from yellowish to blue. When the cell voltage was switched to 0.5 V, the NiO redox reaction occurred, and the color of the device changed to yellowish again. The optical properties of the ECD were also checked by using a spectrophotometer, and the results are illustrated as Figure 5. Two ECDs were investigated in this experiment to realize the effect of ferrocene in the polymeric
![]()
![]() (a) (b)
(a) (b)
Figure 2. (a) The CVs of NiO thin-film electrode in 1 M NaOH aqueous solution; (b) The transmittance spectraofthe NiO thin film at different applied potentials.
![]()
Figure 3. The Schematic diagram of the quasi-solid-state ECD and the photo of the polymeric electrolyte film in the inset.
![]()
Figure 4. The CVs of WO3/polymeric electrolyte/NiO ECD.
![]()
Figure 5. The transmittance spectra of the WO3/polymeric electrolyte/NiO ECD at different applied potentials.
electrolyte. For the ECD without ferrocene, the colored state and bleached state were at −2.5 and 1.9 V. Besides, the transmittance attenuation (ΔT) at 550 nm was 22%. For the ECD with ferrocene in the electrolyte, the ΔT was 37.5%, which was 15.5% higher than that of the ECD without ferrocene. This was because that the introduction of ferrocene changed the safe operation voltage window of the ECD. When the applied voltage was higher than 1.9 V for the ECD without ferrocene, the side reaction occurred and resulted in the damage and instability of the polymeric electrolyte. However, while adding ferrocene into the electrochromic system, the bleaching voltage could be extended to 2.5 V without causing damage to the polymeric electrolyte. Therefore, the NiO thin film could be reduced completely, and thus the transmittance of the ECD could be further improved to 47.5% at 550 nm.
4. Conclusion
In summary, an ECD based on WO3, NiO and a qusai-soild-state polymeric electrolyte has been demonstrated. The device exhibited blue-yellowish electrochromism and could be reversibly switched. After adding ferrocene into the electrolyte, the safe operation voltage window was extended, and the electrochemical and optical properties of the ECD were also improved. The ΔT at 550 nm of the ECD was 37.5%, which was 15.5% higher than that of the ECD without ferrocene. The qusai-soild-state polymeric electrolyte benefits the future application in electrochromicproducts, and there should be more electrochemical active small molecules could be used to enhance the device performance.
NOTES
*Corresponding author.