1. Introduction
Fungal contamination of food and feed by Aspergillus parasiticus or A. flavus and their mycotoxins causes losses particularly in grains and cereal derivatives. Mycotoxins are fungal metabolites that represent the most important category of natural toxins relative to human health and economic impact worldwide [1] [2] . Aflatoxins are a group of compounds produced as secondary fungal metabolites of molds belonging to the genus Aspergillus such as A. flavus, A. parasiticus, A. nomius, A. pseudotamarii, A. bombycis, A. ochraceus and A. australis. The largest proportion of aflatoxins determined in foods is produced by A. flavus and A. parasiticus [1] . Aflatoxin B1 is known as being one of the most potent genotoxic agents and hepatocarcinogens identified, they are classified in the Group 1 of human carcinogenic compounds [1] [3] and the chronic intake of aflatoxins is associated to hepatocellular carcinoma [4] [5] .
Different researchers have shown the antifungal activity of Lactic Acid Bacteria
(LAB)
, their ability to bind mycotoxins in vitro and in vivo, and the capability to reduce the oxidative stress in Balb/c mice caused by the ingestion of aflatoxin B1 and fumonisina B1 mycotoxins [6] -[13] .
Several metabolites produced during the fermentation have shown antifungal effect. These include organic acids (e.g. formic, lactic, acetic, propionic and phenyllactic acids), hydrogen peroxide, diacetyl, short chain peptides and bacteriocins [12] [14] [15] . Other investigations have also shown the yeasts ability to bind aflatoxin B1 in vitro [16] [17] .
Kefir is an antique fermented beverage which is consumed since the Bronze Age in Asia [18] . It is obtained by fermentation of milk with kefir grains which are composed of diverse LAB and yeasts incorporated in a polysaccharide and protein matrix [19] . The antimicrobial properties of the cell-free supernatants (CFS) obtained from kefir products have been associated to the undissociated form of lactic and acetic acids produced during the fermentation process [20] . Previous studies have showed that kefir CFS exhibited antimicrobial activity against E. coli, Salmonella entérica serovar Enteritidis; Shigella flexneri, S. sonnei, Bacillus cereus, B. subtilis, Staphyulococcus aures and Giardia intestinalis [20] -[23] . In addition, CFS obtained from milk or whey fermented with kefir grains showed antifungal activity against A. flavus, Penicillium crustosum, Trichoderma longibrachiatum and Rhizopus macrospores [24] . Other studies with water kefir, a drink obtained from sugary solutions fermented with kefir grains, showed the inhibitory activity of the CFS against A. ochraceus [25] . Recently, a bacteriocin obtained from Lactobacillus paracasei, isolated from kefir grains, inhibited A. flavus, A. niger and P. glaucum [15] .
On the other hand, cheese whey is a by-product of dairy industries, which presents high pollutant characteristics and is produced in high amounts. It is estimated that 450,000 tons of liquid are generated annually in Argentine [26] . Whey disposal has been under consideration for several years. The first option considered was drying it and using it as an additive in food and feed. In this way, the discharge problem was solved but no value was added. As cheese whey has a high content of protein (1% w/v) and lactose (5% w/v), it is appropriate to consider this material as a source of added value compounds and not just as an effluent. The use of whey as a raw material for production of a variety of products (ethanol, biomass, acid lactic, etc.) has been considered by several authors [27] -[31] . Some of the major products obtained from cheese whey are whey protein concentrates [32] . The nutritional and medical characteristics of protein concentrates are generally accepted and their market is increasing. When producing protein concentrates, typically by ultrafiltration, a lactose-rich fraction, cheese whey permeate
(WP)
, is obtained. Due to its composition whey permeate is a strong ambient contaminant [32] .
Kefir fermented products using WP as substrate could give a solution for the ambient contamination and additionally could be used as biopreservative for food and feed with special application against fungal contamination. The objective of this work was to study of the antifungal effect of whey permeates fermented with kefir grains on the growth of A. parasiticus, on the aflatoxin B1 synthesis and the kefir microorganisms protection against the cellular damage produced by aflatoxin B1 on HepG2 cells.
2. Materials and Methods
2.1. Fungal Cultures and Preparation of Conidia Inoculums
A. parasiticus CMUNLP7―isolated from corn (Dra. León at Cathedra of Microbiology-National University of La Plata)―was maintained in soft agar (2 g/L agar in water). The inoculum was prepared by growing the fungi on PDA (Potato Dextrose Agar) slants (
Britania
, Argentina) for 7 days at 30˚C. After incubation, 10 ml of 0.01%
(w/v)
Sodium Lauryl Sulfate
(SLS)
in 1%
(w/w)
sodium chloride solution were added to the tubes and spores were loosened by gently scraping with a spatula, and serial dilutions were made. The number of spores, about 5 × 105/ml was assessed by means of account in a Neubauer chamber [33] [34] .
2.2. Cell Culture
The human hepatocellular carcinoma cell line HepG2 was obtained from the Multidisciplinary Institute of Cell Biology (IMBICE, Buenos Aires, Argentina). These cells have shown to keep many parenchymal cell functions [35] . HepG2 cells were routinely cultured in tissue culture flasks in Dulbecco’s modified Eagle’s medium
(DMEM)
supplemented with 10% fetal bovine serum, penicillin (100 units/ml) and streptomycin (100 mg/ml) at 37˚C in a humidified
(95%)
atmosphere with 5% CO2. The cell culture medium was replaced twice per week. After reaching confluence, the cells were trypsinized and split 1:3. Cells were used for various bioassays according to the corresponding experimental protocol [36] .
2.3. Preparation of Cell-Free Supernatants and Organic Acid Solutions
The kefir grains used -CIDCA AGK1, characterized at the Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CIDCA), UNLP (Garrote et al. 2000) and stored in whole milk at −20˚C―were activated through two consecutive passages of fermentation in ultra-high-temperature-processed (UHT) milk (Sancor, Santa Fe, Argentina). The grains were inoculated into sterile WP at a concentration of 10% (w/v) and incubated at 30˚C until the WP reached a pH of 3.5. The fermentation products were next separated from the grains by passage of the fermentation mixture through a sieve of 1-mm2 mesh size and the microorganisms present precipitated by centrifuging for 15 min at 13,000 g in an Eppendorf 5415D ultracentrifuge (Eppendorf, Hamburg, Germany). The resulting kefir-fermented-whey-permeate cell-free supernatant (KFWP CFS) was sterilized by passage through a microcellulose filter of 0.22-µm pore size (Sigma-Aldrich, St. Louis, USA) and stored at −20˚C until assayed for antifungal activity. The organic acids solutions were prepared with lactic (Carlo Erba 88%, Milan, Italy) and acetic acid (Merck, 99.5%, Darmstadt, Germany). These acids were constituted in sterilized water and mixed in order to obtain different final concentrations. The solutions were stored at −20˚C.
2.4. Fungal Inhibition Assays with KFWP CFS and Organic Acids
The assays were performed on Petri dishes with the basal medium (BM) containing malt extract (1%) (Biokar, Beauvais, France), yeast extract (2%) (Biokar, Beauvais, France) and agar (2%) (Merck, Darmstadt, Germany). The media were autoclaved at 121˚C for 15 min. The agar medium at 45˚C was mixed with the KFWP CFS or with lactic and acetic acids and its mixtures in order to obtain different final concentrations. Twenty milliliters of supplemented medium were plated in Petri dishes (80 mm diameter) and the final pH of the medium was determined with a Hannah pH 211 Microprocessor pH meter.
Control plates were inoculated with the same volume of fungal suspension contained BM medium without any added organic acid. In the assays with KFWP CFS in the media, two different controls were included to investigate the effect of filter-sterilized WP on fungal growth. In the first the BM was used alone, while in the second the BM was supplemented with 70% (v/v) unfermented WP.
Three plates each with additions of KFWP CFS or organic acids were inoculated with 10 µL of the conidia suspensions at 5 × 105 conidia/ml dispensed by micropipetting in the center of the solidified growth medium. The diameter of the circular inoculums obtained was assumed as the colony initial diameter. Inoculated plates were incubated at 30˚C inside plastic boxes containing dishes of water to prevent dehydration. Growth was allowed until the maximum 80 mm diameter, correspondent to the total Petri dish invasion by mycelium; all cultures were kept in the boxes during 30 days in order to determine the total fungal inhibition [37] . Given that filamentous molds grow on solid media forming a circular colony around the initial inoculation zone, colony diameters were measured each daily by placing the Petri dishes on a millimeter scale illuminated from beneath by a light box. Four diameter measures were taken from the center of each colony and the results were calculated from the mean diameter of the replicate colonies [38] .
2.5. Determination of Growth Rate (KD) and Lag Phase (Lag)
Growth rate KD (mm/h), was calculated from the regression slope of colony diameter versus time during the linear growth phase, using the Sigma Plot 9.0™ software. The lag phase (Lag) is the time in hours required for the colony to grow beyond the inoculation zone (typically 5 - 7 mm). This value corresponded to the point on the abscissa where the regression line intersected the horizontal line representing the initial inoculation-zone diameter. Thus,
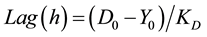 (1)
(1)
where: D0 = diameter of the inoculation zone, Y0 = intersection of the regression line with the ordinate and KD = slope of the regression line (i.e., growth rate; [33] [39] .
2.6. Evaluation of the Fungistatic and/or Fungicidal Effect of KFWP CFS
From the plates of the growth-inhibition experiment where no fungal development occurred, the agar in the central inoculation zone was cut out and placed in 80-mm plates containing unsupplemented MEA in order to assess whether the inhibitory effect on fungal development had been fungicidal or simply fungistatic. The plates were then incubated for 30 days at 30˚C to investigate the fungal-growth capability upon removal from the source of inhibition. This determination was carried out after 7, 15 and 30 days of incubation.
2.7. High-Performance-Liquid-Chromatography Analysis of Lactic and Acetic Acids in KFWP CFS
The concentrations of lactic and acetic acids in the KFWP CFSs were measured by high-performance liquid chromatography (HPLC) in a chromatograph (Agilent Technologies, series 1200, Santa Clara, CA, USA) with an Aminex HPX-87H ion-exchange column (Bio-Rad, Hercules, CA, USA), a mobile phase of 0.009 N H2SO4 (Merck, Darmstadt, Germany), a flow rate of 0.6 mL/min, and an ultraviolet detector measuring at 214 nm [20] . Pure acetic acid (Merck, Darmstadt, Germany) at concentrations of 0.42, 0.83, 1.67, 3.33, 11.70, 25.00, and 66.10 mM and lactic acid (Carlo Erba®, Milan, Italy) at 5.55, 11.10, 44.40, 88.80, 111.00, 155.00 and 209.00 mM were used for the control curves.
2.8. Calculation of the Concentrations of Undissociated Organic Acids
The concentrations of the undissociated acids were calculated by the following equation:
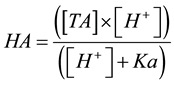 (2)
(2)
where [HA] is the undissociated acid concentration (mM). Ka is the equilibrium constant of lactic (pKa 3.79) or acetic acid (pKa 4.75) and [TA] is the total acid concentration (mM).
2.9. Measurement of Aflatoxin Production by A. parasiticus CMUNLP7 in the Cultures
In order to investigate the effect of KFWP CFS on the mycotoxins production, aflatoxins were extracted from the cultured media in the growth-inhibition experiments. The results obtained with an ELISA kit (Veratox®, Neogen) for total aflatoxins were compared with those determined aflatoxin B1 by HPLC with fluorescence detector in order to asses if a significative difference was observed between the values obtained.
Briefly, each sample was cutted and mixed with a methanol (American Chemical Society certificated-Grade) solution 70% v/v with distilled water and the mixture was filtered through a Whatman N˚ 1 filter paper. Before proceeding to mycotoxins determinations, the samples were passed through VICAM® Aflatoxin immunoaffinity columns (Lot. No. 1610). Total aflatoxins concentration was determined from these samples by means of the immunological kit. Additionally, samples were derivatized for HPLC determination. A purified extract aliquot (200 μL) was mixed with 700 μL of derivatization reagent (trifluoroacetic acid/acetic acid/water 1:7:2) and the solution was heated to 65˚C for 10 minutes in thermostat bath. The derivatized extracts were filtered through 0.45 µm Millipore membrane pore diameter and 50 µL injected into the HPLC. The same procedure was carried out with standard solutions of 75, 150, 300, 500 and 700 µg/mL, which were used to develop the calibration curve. Samples were analyzed by HPLC (Waters 717 plus Autosampler) with fluorescence detection. Chromatographic separations were carried out with a C18 reverse phase column (150 × 4.6 mm, 5 µm particle size, Waters). The mobile phase used was a mixture of water/acetonitrile/methanol (70:17:17, v/v/v) at a flow of 1 mL/min. Fluorescent aflatoxin B1 derivatives were recorded at excitation and emission wavelengths of λ 360 nm and λ 460 nm, respectively and the aflatoxin B1 quantification was performed by measuring the peak area and extrapolating to the calibration curve previously obtained by using Empower software™.
2.10. Protective Effect of Kefir Microorganisms against Aflatoxin B1 Cytotoxicity on HepG2 Cells
WP fermented with kefir grains was obtained as it was described previously. The microorganisms present in the fermented products were precipitated by centrifuging 15 min at 14,000 g in an Eppendorf 5415D ultracentrifuge (Eppendorf, Hamburg, Germany). The pellet was washed twice with PBS buffer (phosphate-buffered saline solution) and once with sterile purified water. Finally, the microorganisms were re-suspended in PBS buffer and the concentration was adjusted to 107 UFC/ml of lactic-acid bacteria (LAB) and yeasts. Kefir microorganisms were pasteurized by heat treatment (72˚C, 30 minutes) and used to study their protective activity on HepG2 cells. Commercial aflatoxin B1 (Sigma Chemical Co, St. Louis, MO, USA) was prepared at different concentrations in DMEM. Cytotoxicity was evaluated by two different methods, one measuring the cell viability and the other the cell damage:
2.10.1. Determination of Succinate Dehydrogenase Mitochondrial Activity as a Measure of Cell Viability
The assay employing 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT) cleavage [40] was used for measuring cell viability. The MTT was used at a dilution of 5 mg/ml in DMEM without phenol red. The tetrazolium salt is metabolically reduced by viable cells to yield a blue formazan product that is measured spectrophotometrically as the optical density at 550 nm. HepG2 cells were seeded in the multiwell-culture plates and grown in DMEM with 10% (v/v) foetal-bovine serum. The cells were washed with PBS and exposed to a mixture of aflatoxin B1 along with total microorganisms of kefir, or to each one separately. Untreated cells were used as a control in all the experiments. The plates were next kept at 37˚C in 5% (v/v) CO2 under growth- arresting conditions (i.e., in serum-free medium) for 24 h. The cultures were washed with PBS and then incubated in the above MTT solution for 3 h at 37˚C. The blue crystals formed within the cell layer were finally dissolved in 200 μl dimethyl sulphoxide and the optical density of the resulting solution measured spectrophotometrically at 550 nm. The mean value for MTT reduction values was compared with the control to determine percent cell viability, which was calculated as ODs/ODc×100 where ODs was the absorbance of the sample and ODc, the absorbance of the control cells without treatment.
2.10.2. Determination of Lactate Dehydrogenase Release as a Measure of Cell Damage
Extracellular lactate dehydrogenase (LDH) activity was evaluated as previously reported [41] . Confluent cultures of HepG2 cells were inoculated with a mixture of aflatoxin B1 and the kefir microorganisms. The tissue-culture plates were incubated at 37˚C in 5% CO2-air for 24 h. LDH was determined in the cell-free supernatant medium of each well by a commercial kit (Wiener Lab, Rosario, Argentina) in which NADH+H+ concentration was measured by optical density at 340 nm in a spectrophotometer (Beckman DU 650, Palo Alto, USA). The total concentration of LDH per well was determined after the monolayer lysis with 3% (v/v) Triton X-100 (Sigma Chemical Co, St. Louis, MO, USA). The percentage of viable cells was calculated as ODs/ODc × 100, where ODs and ODc were the absorbance of the sample and the control, respectively, at 340 nm.
The protective assay was performed in quadruplicate for each sample. At least, three independent experiments were performed for each condition.
2.11. Statistical Analysis
All the growth parameters were analyzed by the SIGMAPLOT 10.0® software. The results of three independent assays are presented as the mean values ± standard deviation (SD). Differences in growth kinetics were tested for significance by the analysis of variance (ANOVA) to determine significant effects at p < 0.05 by means of the STATGRAPHICS Plus 5.1® software. All the experiments were performed at least in triplicate.
3. Results
3.1. Effect of KFWP CFS on the Growth Parameters of A. parasiticus on Solid Medium
We performed fungal-inhibition assays on plates with solid BM in order to study the effect of different concentrations of the KFWP CFS on the fungal-growth parameters (Figure 1). The controls used were unsupplemented BM, BM containing 70% v/v sterile unfermented-WP and BM containing 70% v/v sterile unfermented-WP acidified by HCl. Similar growth rates were observed in all treatments, except in supplemented KFWP CFS of pH 3.5 at 65% v/v medium where growth was not observed. The lag phases were more affected than growth rates and this parameter increased because of the rising of acetic and lactic acids into KFWP CFS.
Table 1 summarizes the growth parameters and the undissociated lactic and acetic acid concentration of KFWP CFS.
![]()
Figure 1. Growth curve of A. parasiticus CMUNLP7 (mm / h) with different proportions of KFWP CFS at pH 3.5 in the BM. ●: BM without KFWP CFS, ●: BM + unfermented WP, ?: BM + WP acidified with HCl 3M, ■: BM + KFWP CFS added at 65% v/v, ■: BM + KFWP CFS added at 62.50%; ♦: BM + KFWP CFS added at 60%, ♦: BM + KFWP CFS added at 55%, ▲: BM + KFWP CFS added at 50% v/v, ?: BM + KFWP CFS added at 25%. Four diameter measures were taken from the center of each colony and the results were calculated from the mean diameter of the replicate colonies. All experiments were performed in triplicate.
![]()
Table 1. Kinetic parameters and undissociated lactic and acetic acids in the inhibition of A. parasiticus CMUNLP7 with KFWP CFS obtained from kefir grains CIDCA AGK1 into solid media.
aLactic and bacetic acids concentrations of were determined by HPLC. Considering final pH media and KFWP CFS dilution, undissociated acids were calculated.
The lag phase of the controls varied between 14.10 and 17.70 h, with these values being statistically equivalent in the two media with WP. Likewise, the respective growth rates in these controls varied between 0.61 mm/h and 0.83 mm/h, observing the higher values in both media containing WP. It suggests that WP stimulates, in some way, fungal growth rate. Furthermore, the pHs of the controls without strong acids (HCl), at 6.35 and 6.28, were only slightly different and the remaining control showed a pH of 4.49. Nevertheless, in spite of pH in the media with WP, the fungal kinetic parameters were similar. These observations indicate that unfermented WP exhibits no growth-inhibitory activity and that the strong acid does not modify fungal growth.
A concentration of 34.08 mM of total undissociated organic acids (at 65% v/v KFWP CFS at pH 3.5 in BM) completely inhibited the fungal growth (Figure 1 and Table 1) in three independent assays. The pH of this treatment was 4.55, similar to that determined in BM acidified with HCl. These results support the idea that the fungicidal effect could not be achieved by the pH drop itself but by the combination of pH and undissociated organic acids. The other treatments with higher KFWP CFS dilutions showed lower concentrations of undissociated organic acids and consequently KD values between 0.64 mm/h and 0.69 mm/h and lag phases between 18.40 h and 75.40 h indicating an inhibitory but not fungicidal effect. It was considered not necessary to compare the inhibitory effect of the strong acid with the CFS at sub-lethal concentrations.
3.2. Evaluation of the Fungicidal or Fungistatic Activity of the KFWP CFS
Samples from the plate growth-inhibition experiment were removed in order to determine whether the action of the KFWP CFS was fungicidal or fungistatic. The antifungal effect exerted by 65% v/v KFWP CFS at pH 3.5 was fungicide. Fungal growth had continued, at least partially, in the remained groups.
3.3. Effect of Lactic and Acetic Acid on the Growth Parameters of A. parasiticus on Solid Medium
Growth-inhibition experiments in plates were performed in which the solid medium contained mixtures of lactic and acetic acids in the absence of KFWP CFS to investigate the effect of the organic acids per se, since they were both present together in the KFWP CFS. Previously, it was determined the minimal concentration of lactic or acetic acids to produce total growth-inhibition. Those concentrations were 460.64 mM and 38.10 mM lactic and acetic acid, respectively.
Table 2 presents the lag phases observed under various mixtures of lactic and acetic acids concentrations. As the results observed in the growth-inhibition experiments with media supplemented with the KFWP CFS, we observed that the most affected fungal growth parameter was the lag phase.
![]()
Table 2. Kinetic parameters and undissociated lactic and acetic acids in the inhibition of A. parasiticus CMUNLP7 with mixtures of acetic and lactic acids into solid media.
aLactic and bacetic acids concentrations of were determined by HPLC. Considering final pH media and KFWP CFS dilution, undissociated acids were calculated.
Comparing the data obtained in Table 1 and Table 2, we observed that mixtures of undissociated lactic and acetic acids at concentrations similar or higher than those found in KFWP CFS, triggered shorter lag phases. In order to produce significant increase in fungal lag phases, it was necessary to use mixtures with total undissociated acids between 24.06 - 37.71 mM to produce lag phases between 47.02 - 25.80 h, respectively, whereas in KFWP CFS supplemented media (at 50%) with total undissociated acids 16.45 mM triggered a lag phase of 36.90 h. This means that it is necessary total undissociated acids in the mixtures 1.29 times higher than those obtained with KFWP CFS in order to obtain a fungicide activity.
3.4. Effect of Lactic and Acetic Acids on Aflatoxin Production by A. parasiticus CMUNLP7
We studied the effect of lactic and acetic acids on aflatoxin production by A. parasiticus CMUNLP7 (Figure 2). Aflatoxin production was measured in BM supplemented with KFWP CFS at different concentrations (25%, 50% and 65% v/v). We measured aflatoxin production in these conditions by HPLC and immunologic test (Veratox®, Neogen). The lactic and acetic acids concentration in supplemented media was determined by HPLC and undissociated total acids concentrations was calculated. Figure 2 shows similar results with both methods (HPLC, red circles and Veratox, green circles).
We observed through these techniques that no toxic production was detected in KFWP CFS supplemented medium at 65% (v/v)-fungicide condition (34.08 mM undissociated total acids). We noticed that in media with non-fungicide-activity, aflatoxin production by A. parasiticus CMUNLP7 was slightly modified with the lactic and acetic acid concentration. We found that aflatoxin produced in unsupplemented BM (between 0 - 31.18 ppb, data not shown), was lower than that assessed in media supplemented with WP (104.02 - 120.93 ppb) (Figure 2) It suggests that WP stimulates aflatoxin production by the studied fungi.
Figure 2 shows that aflatoxin production increased as a function of lactic and acetic acids mixtures added to basal medium.
No aflatoxins were detected in media added with mixtures of lactic and acetic acids at fungicide concentration (48.83 mM). In mixtures of undissociated total acids between 32.82 mM and 37.71 mM media, fungal growth was observed and aflatoxin production was detected between 231.06 ppb and 210.05 ppb.
![]()
Figure 2. Aflatoxin production by A. parasiticus CMUNLP7 in media with different concentrations of undissociated total acids. ●: Aflatoxin B1 produced into solid media with KFWP CFS measured by HPLC. ●: Aflatoxin B1 produced into solid media with mixtures of lactic and acetic acids measured by HPLC. ●: Total aflatoxins produced into solid media with KFWP CFS measured by inmulogic test Veratox®. All experiments were performed in duplicate.
3.5. Effect of Kefir Microorganisms against Aflatoxin B1 Cytotoxicity
Once determined the antifungal effect, we decided to study if kefir microorganisms could exert a protective effect against the cytotoxic effect of aflatoxin B1 on a HepG2 cell line. The damage exerted by aflatoxin B1 against HepG2 was evidenced by the MTT assay that determinates mitochondrial dehydrogenase activity as a maker of cell viability (Figure 3). Different amounts of aflatoxin B1 produced a dose-dependent decrease in cell viability. After 24 h of exposure, 294.09 ppb of aflatoxin B1 caused a marked decrease of the number of viable cells to 50% of the control level. Figure 3 shows that kefir microorganisms were effective in protecting HepG2 cells from the cytotoxic effect caused by aflatoxin B1.
In order to improve and corroborate the data obtained with the MTT assay we studied the cell toxicity by the lactate dehydrogenase (LDH) assay. Lactate dehydrogenase release is an indicator of membrane leakage as a result of the cell damage (Figure 4). With the LDH assay different amounts of aflatoxin B1 produced a dose- dependent increase in cell damage. Figure 4 shows that kefir microorganisms were effective in protecting HepG2 cells from the citotoxic effect caused by aflatoxin B1.
4. Discussion
Milk fermented by kefir grains has been reported to contain, in general, lactic and acetic acids, acetaldehyde, ethanol, carbon dioxide (in equilibrium with carbonic acid), B vitamins, and hydrocarbons such as diacetyl [20] [42] . In our experiments we reported for the first time the presence of lactic and acetic acid in whey permeates fermented with kefir grains. The supernatants obtained from the fermented products showed antifungal activity being the most inhibitory the one obtained at pH 3.5 at concentration of 65% v/v. We need to emphasize that these experiments were conducted with CIDCA AGK1 kefir grains grown in WP and that the latter is an effluent of the dairy industry. These results suggest that CIDCA AGK1 kefir grains exhibit an impressive growth capability in a low-cost industrial by-product while at the same time is a promising biopreservative.
Recent investigations have showed the antifungal activity of kefir fermented products. Cell free supernatants obtained from milk fermented with kefir grains were antifungal against A. flavus and Fusarium graminearum. Whereas these researchers reported a complete growth inhibition of A. flavus employing the CFS at a concentration of 10% (v/v) in the culture medium [43] ; in our experiments, we needed a concentration of 65% (v/v), in order to inhibit the fungal growth. Other investigations have showed that cell free supernatants obtained from whey fermented with kefir grains reduced the germination of the conidia of Rhizopus sp., A. fumigatus, A. terreus, A. flavus and A. parasiticus. Whey contains proteins, lipids and lactose apart from different minerals, being
![]()
Figure 3. Mitochondrial dehydrogenase activity related to aflatoxin B1 in DMEM media with (●) and without (●) total microorganisms of kefir on HepG2 cells.
![]()
Figure 4. Lactate dehydrogenase (LDH) activity related to aflatoxin B1 in DMEM media with (●) and without (●) total microorganisms of kefir on HepG2 cells.
a rich medium to kefir growth, a source of added value compounds and not just an effluent [24] . In our studies, we used WP, a nutritional poor medium obtained from whey deproteinization. In spite of growing in poor medium, kefir grains had the ability to grow on it and to produce a fermented product with antifungal activity. Recently, other authors have shown the antifungal effect of different supernatants obtained from a sucrose solution fermented with water kefir grains against A. ochraceus [25] .
In the mixture of organic acids with growth-inhibitory and fungicidal capabilities (55.56 mM lactate and 19.10 mM acetate), 92.20% of the acetic acid is in its undissociated form, which species is able to penetrate the fungal membranes through hydrophobic interactions, while in comparison only 56.29% of the added lactic acid remains undissociated. These data further corroborate the function of lactic acid as being principally to reduce the pH and the main inhibitory function of the acetic acid. In general, lactic, acetic, and propionic acids interact with the fungal intracellular membranes in a similar way to neutralize the proton electrochemical gradient, but the effect of acetic and propionic acids depends, often on the reduction in pH effected by lactic acid [44] . Our data showed that the minimal inhibitory concentration value assessed for the mixture of lactic and acetic acids corresponded to 12.06% and 50% of the respective individual minimal inhibitory concentration. These percentages indicate that acetic acid is the main agent responsible for the antifungal activity, with lactic acid serving mainly to reduce the pH of the culture medium down to acidity in the neighborhood of 4.75, the value of the pKa of acetic acid. Previous studies have reported synergistic effects between lactic and acetic acid [34] . Strong similarities were found among the synergistic effect obtained in our investigation and that obtained previously with acid mixtures employed to inhibit A. flavus, where the concentration of acetic acid was 75% of its individual minimal inhibitory concentration, while the corresponding concentration for the lactic acid in the mixture was only 21.8% of its minimal inhibitory concentration value [34] .
We compared the total amount of undissociated acids in the acids mixtures with those corresponding to the KFWP CFS with fungicidal activity. In the acids mixtures, the necessary undissociated acids amount was 1.29 times higher than that obtained with KFWP CFS. Lactic and acetic acids have been considered the main acids in the kefir fermentation products that exert microbial inhibition [20] . Nevertheless, it is possible that other compounds are present in the CFS that could have a synergistic effect with lactic and acetic acid. Recent studies have reported the production of other compounds by lactic acid bacteria isolated from other substrates. Nevertheless there are no reports of these compounds in kefir products. Some lactic acid bacteria can produce phenyl lactic acid but in lower concentrations that lactic and acetic acid, which implies that the antifungal effect depends on the synergistic effect among all the acids present within the fermentation products [14] . Other antifungal metabolites are present in fermentation products from lactic-acid bacteria isolates. These are lactic, acetic, and phenyl lactic acids and a peptide produced by Lactobacillus fermentum [12] . Recent studies has shown that a proteinaceous compound, produced by Lactobacillus paracasei subsp. tolerans FX-6 isolated from Tibetan kefir, showed antimicrobial activity against E. coli, S. aureus, B. thuringiensis, S. enterica, S. dysenteriae, A. flavus, A. niger, Rhizopus nigricans and Penicillium glaucum [15] . The consideration mentioned above suggests that the fermented products could contain additional metabolites such as phenyl lactic acid, propionic acid and low molecular weight peptides that improve the lactic and acetic acid antifungal activity [6] [9] -[12] [15] . Our results showed that the kefir fermented products are promising as biological controls and that further studies must be done in order to determine the metabolites interaction in the antifungal activity.
In order to assess the aflatoxin detection we compared two methods. High performance liquid chromatography (HPLC) is a very accurate analytical method currently used for aflatoxins determinations [45] that requires nor only the extraction steps but the aflatoxin purification and derivatization. The advantages of using the enzyme-linked immunosorbent assay ELISA are a reduction in the assay time, a simplified sample extraction and specificity for the toxin in question [46] . Nevertheless, the disadvantage of these kits lies in the fact that they are for single use, which can increase costs of bulk screening. Additionally, the competitive ELISA employed determines only total aflatoxins and not AFB1 exclusively [47] . Nevertheless, the analyses of the aflatoxin B1 extracts conducted with the commercial ELISA kit and the HPLC method performed in our laboratory gave similar results. This proved the validity of the methods used and the possibility of using both techniques in the experiments developed with the basal medium. Our results are in concordance with those realized with aflatoxin M1 in milk. Similar results were obtained in the AFM1 concentration detected by both methods, thereby confirming that ELISA is a very effective detection method, and that both are valid methods [48] .
Once we had found the fungal inhibition by pure acids or by KFWP CFS, we assessed the effect of the fermented products on the aflatoxin B1 production. No aflatoxin production was observed with KFWP CFS fungicide concentration (65% v/v). The addition of sterile whey permeate to the control media increased the aflatoxin production in relation with the basal medium without any addition. The aflatoxin production in supplemented media with lower concentration of KFWP CFS, was similar or slightly upper than WP supplemented medium. On the other hand, lactic and acetic acids mixtures stimulated significantly aflatoxin production by A. parasiticus.
On the other hand, the cytotoxic effects of aflatoxin B1 on HepG2 cells was determined. At 24 h of exposure, 294.09 ppb of AFB1 (0.94 µM) caused a marked decrease of the number of viable cells to 50% of the control level. The LC50 was estimated at that concentration. Our results are in line with previous studies where IC50 value was 1.0 µM of aflatoxin B1 in the same cell line [49] . Moreover, we demonstrated that kefir microorganisms protect HepG2 cells against the cytotoxic effects of aflatoxin B1. Our results coincide with those obtained by [50] with lactic acid bacteria. Lactobacillus acidophilus and Bifidobacterium animalis detoxify ochratoxin A and Patulin respectively and consequently reduce the cytotoxic effect of these mycotoxins on HepG2 cells. These promising results indicate a new property of kefir microorganisms and improve their probiotic characteristics.
5. Conclusion
The presence of KFWP CFS at a pH 3.5 in high concentrations exhibited a fungicidal capability against A. parasiticus CMUNLP7. At this condition the concentration of undissociated lactic plus acetic acid is lower than that observed in the mixtures of both acids, suggesting the presence of other metabolites produced by kefir microorganisms that improve the antifungal activity. In these conditions aflatoxin production was not observed. The kefir microorganisms showed protective ability against cytotoxic effect of aflatoxin B1 on HepG2. This is the first report about the protective effect of the kefir microorganisms on the human hepatocellular carcinoma HepG2 cells against AFB1 cytotoxic effect. Further studies in vivo and in vitro must be realized in order to corroborate these promising results on the probiotic properties of the kefir microorganisms.
Acknowledgements
The authors acknowledge Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina), Universidad Nacional de La Plata (UNLP, Argentina), Centro de Investigación y Desarrollo de Fermentaciones Industriales (CINDEFI) and Centro de Investigación en Criotecnología de Alimentos (CIDCA) for financial support. Raùl Gamba is recipient of a postdoctoral fellowship from CONICET, T. Alconada, A. Astoreca and G.L De Antoni are researchers of CONICET. A. León Peláez is professor at the Universidad Nacional de La Plata.