1. Introduction
Food crops have occupied an important place in human nutrition as they remain the major sources of calories and proteins for a large proportion of the world population. Legumes and cereals, the main plant sources of proteins in human diet, are in addition rich in dietary fibre and carbohydrates [1] . Minor compounds of legumes are lipids, polyphenols, and bioactive peptides [2] . Legumes will therefore continue to play an important part in diets in the foreseeable future. Legumes provide a good source of protein (18% - 35%), and supplement cereals not only for protein but also for minerals and vitamins of B complex. This is particularly important when refined cereals such as white wheat flour are used in a poor diet with few supplementary foods.
Legumes have been promoted as a source of protein in countries with high rates of protein-energy malnutrition. Cowpeas are abundant in these countries and contain proteins, carbohydrates, water soluble vitamins and minerals [3] . The cowpea (Vigna ungiculata L. Walp) is a grain legume believed to have originated in Africa and Asia [4] , and is widely cultivated in the tropics [5] . As a legume, cowpeas are rich and low-cost sources of proteins and nutrients [6] and they form part of staple diet in most African and Asian countries. Cowpeas are consumed as boiled vegetables using fresh or rehydrated seeds or processed into flour to make other food products. Major limiting factors to the utilization protein quality include poor digestibility, deficiency of sulphur amino acids and presence of anti-nutritional factors such as trypsin inhibitors, oligosaccharides and phenolic compounds [5] [7] [8] . Thus cowpea must be adequately processed, especially when it is used as a main component of the diet of young children. Proteins are major components of legume seeds. Their nutritional and functional properties dramatically affect the overall quality of the seed and its technological performance [9] . The crude protein of cowpea seeds ranged from 18% to 35% based on variety and the protein of cowpeas was found deficient in methionine and tryptophan [10] . The digestibility of the diet containing cowpea protein isolate was 87% and showed positive nitrogen balance (NP = 0.5) and a net protein retention (NPR) of 0.7 [11] . This study aims to investigate the nutritive value of cowpea seeds, as one of the legumes widely consumed in developing countries. Further studies are needed for a multi-parameter approach in quality evaluation [12] and on the effect of processing on finished products [13] .
2. Materials and Methods
Samples: Dehulled cowpea (Vigna unguiculata L. Walp) of white coloured seed (Figure 1) was brought from the local market at Wad Medani city, Gezira state, Sudan. The seeds were stored in polyethylene bags at room temperature.
![]()
Figure 1. (a) Whole cowpea seeds; (b) Dehulled cowpea seeds; (c) CPII: cowpea protein isolate prepared by isoelectric precipitation; (d) CPIM: cowpea protein isolate prepared by micellization precipitation.
Seeds were ground to a flour passing 35 mesh, defatted by soaking in petroleum ether (BP. 40˚C - 60˚C) for 48 h with several changes of the solvent. The solvent decanted and defatted flour was then air dried over night at (27˚C) and kept in clean bottles.
Preparation of cowpea protein isolate (CPI) by isoelectric precipitation (CPII): CPII was prepared from cowpea seed flour as shown in Figure 2 following the method described by Thompson [with slight modifications [14] [15] .
The insoluble matrices were separated by refrigerated centrifugation at 4 × 103 g for 20 min and discarded. Extraction and centrifugation procedures were repeated. The supernatant was adjusted to pH 4.0 with 1.0 N HCl and stirred at room temperature for 20 min; followed by refrigerated centrifugation (4000 g, 20 min). The precipitate was washed by distilled water several times to free it from salt; neutralized to pH 7.0 using 1.0 N NaOH and left over night in refrigerator (4.0˚C). The isolate was freeze dried, ground into powder using a ceramic mortar and pestle and stored in a desiccators.
![]()
Figure 2. Preparation of cowpea protein isolates by isoelectric (CPII) and micellization precipitate (CPIM).
Preparation of cowpea protein isolate (CPI) by micellization precipitation (CPIM): CPIM was prepared using micella method [16] as presented in Figure 2. The defatted seed flour was suspended in NaCl 1.0 N solution in a 1:10 (w/v) ratio, then stirring for 2 h at room temperature. The suspension was centrifuged at 3000x g for 30 min and then the residue was extracted again as described above. The combined supernatant was diluted ten folds by distilled water and left to stand at (4˚C), in refrigerator, for 18 h.
Chemical composition: Cowpea seed flour and protein isolate composition were determined following methodology for total nitrogen (Kjeldahl), fat (Soxhlet), carbohydrates, moisture and ash (gravimetrically) and crude fibre by a chemical-gravimetric method [17] and the means reported on dry weight basis.
Amino acid analysis: Amino acids analysis was performed on (DDCF) and protein isolates using amino acid analyze chromatography method [18] and quantified after reaction with ninhydrin. Each sample was hydrolyzed with 6 N HCl at 110˚C for 24 h. The amino acid composition was calculated considering the highest value for each amino acid. Sulfur-containing amino acids were determined after performing acid oxidation.
Biological values of cowpea seed flours and isolates were determined on the basis of its amino acid profile. Chemical score was calculated [19] . The content of each essential amino acid in test protein was expressed as a percentage of the content of the same amino acid in a standard protein. Amino acid was calculated using the FAO/WHO [20] reference pattern. The amino acid showing the lowest percentage was called the “limiting amino acid” representing the chemical score. Essential amino acid index (EAAI) was calculated according to [21] using the amino acid composition of the whole egg protein [22] .
Protein efficiency ratio (PER) was estimated according to the following regression equation [23] .
Predicted protein efficiency ratio (P-PER)
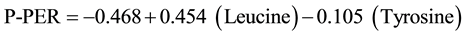
The net protein value (NPV) was calculated by multiplying the lowest amino acid score by the percent of protein divided by 100.

3. Results and Discussion
Chemical composition: Chemical composition of dehulled defatted cowpea flour (DDCF) and protein isolates (CPII and CPIM) (Table 1). The whole (WCF) and dehulled defatted (DDCF) cowpea seed flour contained 22.30% - 26.73% protein, 2.10% - 2.30% fat, 4.10% - 1.02% fibre, 3.77% - 3.87% ash and 60% - 59% carbohydrates, respectively (on dry weight basis) as major components. The CPIM showed significantly (p < 0.05) higher protein than the CPII but the fat, ash and crude fibre contents of both isolates were similar. Protein isolate by micellization techniques, the protein association was favored when ionic strength (µ) of the extract sample was reduced [24] [25] . This may be the main reason this isolate had higher protein content. Some salts bind to protein when present at relatively high concentrations, the increase in net charge due to ion binding results in increased repulsive forces and destabilization of the protein [26] .
![]()
Table 1. Proximate composition of whole cowpea flour (WCF), dehulled defatted cowpea flour (DDCF) and protein isolates (CPII) and (CPIM) % dry basis.
Means in the same raw with different letters are significantly different (p < 0.05). Means ± Standard deviation of triplicate analysis. LSD: Least significant differences; CPII: Cowpea protein isolate prepared by isoelectric point precipitation; CPIM: Cowpea protein isolate prepared by micellization precipitation.
Amino acid composition: The protein quality or the nutrient value of food depends on its amino acid content and on the physiological utilization of specific amino acid after digestion, absorption and utilization. The two prepared protein isolates are rich in isoleucine, leucine, lysine and total aromatic amino acids (tyrosine and phenyl alanine) (Table 2). Similar observations were reported [27] . Methods based on in vitro (chemical and amino acids bioassay) for assessment of protein quality is important.
Amino acid composition of the DDCF and protein isolates CPII and CPIM are reported as g/16 g N comparing to the [20] (Table 2). The protein isolates are rich in leucine the values were 8.8 and 8.9 g/16 g N for CPII and CPIM respectively. This result was similarly reported [15] 8.0 and 8.4 g/16 g N for pea and faba bean proteins isolates. Methionine was the most essential amino acids in both protein isolates (CPII and CPIM). The protein isolates showed, in general higher total essential and nonessential amino acid levels than their own original seeds (Table 3). Cystine is most limiting sulphur containing amino acid in cowpea flour (Table 4). This is a general nutrient problem with most of the legume seeds. In fact, both isolates showed higher levels of total sulphur and aromatic amino acids compared to original seeds. The essential/non essential ratios showed slight increase in the protein isolates profile compared to the ratio in their respective seeds. On the basis of chemical scores both isolates have higher chemical scores than the legume seeds (Table 4). Net protein value of protein isolate CPII was higher than isolate CPIM.
The low (leucine:isoleucine) ratio in both cowpea protein isolates was desirable because it leads to amino acid balance in cereals that are already high in leucine and low in tryptophan and isoleucine.
Both isolates (CPII and CPIM) had higher content from non essential amino acids than cowpea flour Figure 3. However, the hydrophobic amino acids (leucine, isoleucine and valine) were more abundant in CPIM (23.66) than in CPII (22.33).
![]()
Table 2. Amino acid composition of dehulled defatted cowpea flour (DDCF) and cowpea protein isolates (CPII and CPIM).
CPII = Cowpea protein isolate by isoelectric point precipitation; CPIM = Cowpea Protein Isolate by micellization precipitation.
![]()
Figure 3. Evaluation of protein quality based on amino acid composition in dehulled defatted cowpea flour (DDCF) and protein isolates (CPII and CPIM). EAA: Essential amino acids; POAA: Positively amino acids; NPAA: Non polar amino acids; NEAA: Non essential amino acids; NEAA: Negatively charge amino acids; PAA: Polar amino acids; ARAA: Aromatic amino acid.
![]()
Table 3. Classification of amino acids (g/16 g) of dehulled defatted cowpea flour (DDCF) and protein isolates (CPII) and (CPIM).
CPII = Cowpea protein isolate by isoelectric point precipitation; CPIM = Cowpea protein isolate by micellization precipitation.
![]()
Table 4. Estimation of nutritional quality of dehulled defatted cowpea flour (DDCF) and protein isolates (CPII and CPIM) based on amino acids composition.
DDCF = Dehulled defatted cowpea flour; CPII = Cowpea protein isolate by isoelectric point precipitation; CPIM = Cowpea protein isolate by micellization precipitation; MEAA = Michel essential amino acids index; NPV = Net protein value.
The basic amino acids (BAA) were found greater than total acidic amino acids (AAA) (aspartic amino acid not detected in all samples), indicating that the protein is probably basic in nature.
4. Conclusions
Whole cowpea flour (WCF), de-hulled defatted cowpea flour (DDCF) and protein isolates obtained by isoelectric (CPII) and micellization (CPIM) precipitation have shown some changes in the proximate composition. Crude fibre decreased from 4.1% in WCF to 1.02% in DDCF, while crude protein increased from 22.3% in WCF to 26.75% in DDCF. Protein isolates (CPII and CPIM) showed 75% and 76% protein content and a decrease in carbohydrate content from 59.78% to 13%.
The first limiting amino acid was cystine (0.032 g/16 g nitrogen) for DDCF and threonine (5.60 and 4.18 g/16 g nitrogen) for CPIA and CPIB, respectively. Essential amino acids of CPII and CPIM were found in an acceptable level in comparison with reference protein. Both protein isolates were higher than FAO/WHO in lysine content. The values were 22.99 and 15.78 g/16 g N in protein isolates CPII and CPIM respectively. Methionine was found to be the most concentrated essential amino acids in both protein isolates (CPII and CPIM), with values ranging from 27.22 to 30.60 g/16 g N respectively, while lysine was the most abundant essential amino acid in DDCF (4.28 g/16 g N). Net protein values (NPV) were 17.62 for DDCF and (8400 - 7942) for CPII and CPIM respectively. Chemical scores for both isolates were above 100.
A mixed food of legumes and cereals, particularly in developing countries, can compensate deficiencies or a low level of lysine and sulphur amino acids, in cereals and grain legumes, respectively.