1. Introduction
Sea cucumbers, also known as holothurians, are one of the most important seafood products in China, Japan and Singapore, where the tradition relies on eating the sea cucumbers’ body wall, known as bêche-de-mer. Further- more, the use of sea cucumbers have been raising because from a nutritional point of view, these organisms have high protein and low fat and are particularly rich in essential fatty acids that are responsible for preventing heart and degenerative diseases [1] -[7] .
Recent studies have associated the use of sea cucumbers to the development of new therapies for the treatment of diseases, since these marine organisms have a variety of bioactive compounds with important functional activities. Moreover, various species of sea cucumbers have been used in clinical trials, since they exhibit anticoagulant, anti-inflammatory, antimicrobial, antioxidant, antitumor, tissue regeneration, among others capacities [5] [6] .
The development of laboratory assays in vitro has gained increasing importance in assessing the ability of sea cucumbers to different microorganisms and cell lines. Methanol, n-hexane and dichloromethane are the most used solvents in sea cucumber studies, revealing the presence of saponins and other chemical compounds [4] [8] -[11] .
Stichopus regalis (Cuvier, 1817), also known as Parastichopus regalis, is a holothurian species that belongs to the family Stichopodidae. It is a benthic species that can be found beyond 50 m and has a wide geographical distribution that includes the northwestern Mediterranean, eastern Atlantic from the south of the Canary Islands to the north of Ireland, and western Atlantic, Antilles and Gulf of Mexico. Stichopus regalis is the only species of Stichopodidae family to be found in the Mediterranean Sea and it is a much appreciated product in the Balearic Islands and Catalonia (NW Mediterranean), reaching 130 ?per kg and being known under the local names of “espardenya”, “llongo”, “llonguet” or “sola” [12] [13] .
To our knowledge, this is the first work combining the study of the biotechnological potential of this species and their important nutritional value by analyzing the lipid profile.
This study aims to provide information about the antioxidant, antimicrobial and antitumor potential of Stichopus regalis extracts and to analyze the total fat content and fatty acid profile of this economical important sea cucumber species.
2. Materials and Methods
2.1. Animals
A total of 15 individuals were collected by commercial fishermen from Peniche (Portugal) between 50 m and 100 m deep, in September of 2012. The samples were cleaned and stored in proper plastic recipients with sea water and transported to the Aquaculture Laboratory of the School of Tourism and Maritime Technology. Each sea cucumber was weighed and a longitudinal incision was made along the dorsal surface and the coelomic fluid and gonads were removed. Then the samples were stored at −80˚C until further use.
2.2. Preparation of the Extracts
The extracts were prepared according to the adapted method by Mayachiew& Devahastin (2008) [14] . Lyophilized holothurians were ground with a mixer grinder to make a powder which was sequential extracted with 1:4 biomass:solvent ratio with methanol and dichloromethane at constant stirring for 12 hours. The solvents were evaporated in a rotary evaporator (Laborota 4000, Heidolph) at 40˚C and the extracts were then solubilized in dimethyl sulfoxide (DMSO) and stored at −20˚C until further use.
2.3. Antioxidant Capacity
2.3.1. Total Phenolic Content (TPC)
The total phenolic content was determined using Folin-Ciocalteu method, according to the adapted work of Yu et al. (2002) [15] . In a microtube it were added 790 µL of distilled water, 10 µL of sample and 50 µL of Folin-Ciocalteu reagent. After 2 minutes, 150 µL of sodium carbonate, Na2CO3 20% (w/v) was added. Tubes were then vortexed and held at room temperature for 1 hour. Absorbance of the blue colored solution was recorded at 755 nm against blank containing water instead of the Folin-Ciocalteu reagent. Total phenolic content was calculated as gallic acid equivalents using calibration curves prepared with gallic acid standard solutions. All measurements were carried out in triplicates.
2.3.2. 2.2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
The DPPH free radical scavenging activity was evaluated by the described method of [16] . A solution of DPPH 0.1 mM in ethanol was prepared. Then, it was added 10 µL of sample to 990 µL of DPPH solution. The mixture was vortexed for 1 min and allowed to stand at room temperature in the dark for 30 minutes. A blank was prepared by adding 10 µL of extracts to 990 µL of ethanol. The control was 990 µL of DPPH solution with 10 µL of DMSO. Changes in the absorbance were measured through spectrophotometric reading at 517 nm. All measurements were carried out in triplicates. The radical scavenging activity was expressed as the inhibition percentage and was calculated using the following formula:
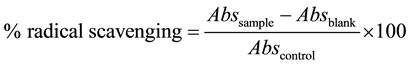
2.3.3. Oxygen Radical Absorbent Activity (ORAC)
The oxygen radical absorbent capacity (ORAC) assay was performed as described by Dávalos et al. (2004) [17] as follows: the reaction was carried out in 75 mM phosphate buffer (pH 7.4), and the final reaction volume was 200 µL. Sample (20 µL) and fluorescein (120 µL; 70 nM, final concentration) were placed in the well of the microplate. The mixture was pre-incubated for 15 min at 37˚C. AAPH solution (60 µL; 12 mM, final concentration) was added rapidly using a multichannel pipet. The microplate was immediately placed in the reader and the fluorescence recorded every minute for 240 min. The microplate was automatically shaken prior to each reading. A blank using phosphate buffer instead of the fluorescein and eight calibration solutions using Trolox (1 - 8 µM, final concentration) as antioxidant were also carried out in each assay. All the reaction mixtures were prepared in duplicate, and at least three independent assays were performed for each sample.
Antioxidant curves (fluorescence versus time) were first normalized to the curve of the blank corresponding to the same assay by multiplying original data by the factor fluorescenceblank,t = 0/fluorescencesample,t = 0. From the normalized curves, the area under the fluorescence decay curve (AUC) was calculated as:
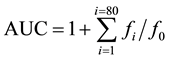
where f0 is the initial fluorescence reading at 0 min and fi is the fluorescence reading at time i. The net AUC corresponding to a sample was calculated by subtracting the AUC corresponding to the blank. Regression equations between net AUC and antioxidant concentration were calculated for all the samples. ORAC values were expressed as Trolox equivalents (TE) by using the standard curve calculated for each assay. Final results were expressed in µmol TE/g of extract.
2.4. Antimicrobial Activity
The antimicrobial activity of Stichopus regalis extracts was evaluated against eight microorganisms: Escheri- chia coli (ATCC 25922 and ATCC 10536), Pseudomonas aeruginosa (ATCC 27853), Bacillus subtilis (ATCC 6633) and Salmonella enteritidis (ATCC 13076) were cultured at 37˚C in Luria-broth (LB); Staphylococcus au- reus (ATCC 25923) was cultured at 37˚C in Trypticase Soy Yeast Extract medium (TSYE) and Saccharomyces cerevisiae (ATCC 9763) and Candida albicans (ATCC 10231) were cultured in Yeast Extract Peptone Dextrose (YPD) medium at 30˚C and 37˚C, respectively. All mediums were obtained from Merck (Darmstadt, Germany).
The antimicrobial assays were performed in 96 well plates, where it was added 193 µL of medium, 5 µL of microorganism inoculum and 2 µL of test samples per well and then incubated. Chloramphenicol (1 mg∙mL−1) and amphotericin B (300 µg∙mL−1) (Sigma Aldrich, Oakville, Canada) were used as positive controls and a blank for each sample was prepared. All samples were sterile filtered and the assays were performed in eight independent experiments under sterile conditions.
The ability of extracts to inhibit microbial growth was evaluated through spectrophotometric analysis at 600 nm. Results were expressed in percentage of control by the following equation:
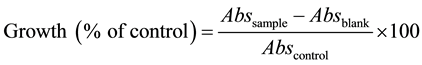
where Abssample corresponds to the absorbance of microbial growth in the presence of the sea cucumber extracts, Absblank corresponds to the absorbance of the extracts in the respective medium and the Abscontrol corresponds to the absorbance of microbial growth without the extracts.
Fractions that showed the highest potential in reducing microbial growth were also evaluated through dose- response analysis in order to determine the IC50 values.
2.5. Cytotoxic and Anti-Proliferative Activities
The cytotoxic potential (cell proliferation and viability) was tested on MCF-7 (breast carcinoma) and HepG-2 (human hepatocellular carcinoma) cell lines. Cells were cultured in RPMI 1640 medium supplemented with 10% of fetal bovine serum (FBS) and 1% antibiotic/antimycotic for HepG-2 cell line and 10% of fetal bovine serum (FBS), 1% antibiotic/antimycotic, MEM (non-essential amino acids), sodium pyruvate at 1 mM and human insulin at 10 µg∙mL−1 for MCF-7 cell line. The cell lines were maintained in culture in a CO2 incubator at 5% CO2, 95% humidity and a constant temperature of 37˚C.
The cell proliferation and cell viability studies were evaluated through the method reported by [18] [19] . For cell proliferation assays, after 36 hours of the cells being seeded in 96-well plates, it was added 100 µL of medium with serum and 100 µL of medium without serum with the sea cucumber extracts. All fractions tested were incubated at 1 mg∙mL−1 for 24 hours. In cell viability assays, after the cells reach the total confluences, it was added 200 µL of the extracts dissolved in medium without serum. All fractions were incubated at 1 mg∙mL−1 for 24 hours.
A dose-response assay (IC50) was performed for the fractions that showed the highest potential. The effects were revealed by the MTT and Calcein-AM methods. The results were presented as percentage of control, being calculated by the following equation:

where Fsample corresponds to the fluorescence/absorbance of the sea cucumber extracts plus cell lines and Fcontrol corresponds to the fluorescence/absorbance of DMSO (same % of the extracts) plus cell lines.
2.6. Quantification of Total Fat Content and Fatty Acid Profile
The total fat content was quantified following the modified [20] procedure. 10 g of homogenized sample was weighed and dissolved in 30 mL methanol:chloroform (2:1 v/v) solution. After, 4 mL of NaCl, 10 mL ultrapure water, and 10 mL chloroform were added and the solution was stirred for 5 min at 400 rpm. The mixture was maintained in an ultrasonic bath (45 KHz, 130 W) at 25˚C for 10 min. A filtration under vacuum was made and the filtrate was transferred to a separating funnel and allowed to settle for phase separation. The organic bottom layer was dried on an anhydrous sodium sulfate column, recovered into a vial and evaporated at 40˚C. The total fat content was calculated according to the following equation:
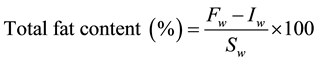
where Iw is the initial weight of the vial (g), Fw is the final weight of the vial (g), and Sw is the sample weight (g).
The fatty acid profile was performed by the adapted work of [21] . 300 mg of crude fat were dissolved in 5 mL acetyl chloride: methanol (1:19 v/v) and heated at 80˚C for 1 h. After, 1 mL of ultrapure water and 2 mL n-heptane were added and the solution was centrifuged at 1500 × g for 5 min. The organic upper phase was analyzed by GC, using a Finnigan Ultra Trace gas chromatograph equipped with a Thermo TR-FAME capillary column, an auto sampler AS 3000 and a flame ionization detector (FID) to quantify the fatty acid methyl esters.
2.7. Statistical Analysis
A 1-way ANOVA followed by a post hoc Dunnett test was used for statistical analysis [22] . The results were considered statistically significant at a significant level of 5%, using IBM SPSS Statistics 20 software.
3. Results and Discussion
3.1. Antioxidant Activity
No significant antioxidant activity was detected in the three methods under study for both organic fractions of Stichopus regalis (data not shown).
3.2. Antimicrobial Activity
In order to evaluate the antimicrobial potential of Stichopus regalis, the methanolic and dichloromethane fractions were tested against seven microorganisms and the positive results are summarized in Figure 1(a) and Figure 1(b). The methanolic fraction showed the highest potential on S. cerevisiae growth reduction (Figure 1(a)), with a percentage of 62.80% ± 0.02% and the dichloromethane fraction presented a percentage of growth reduction of 60.21% ± 0.01%. Concerning C. albicans (Figure 1(b)) the methanolic fraction also showed the highest potential with an inhibition of 86.20% ± 0.03%. In the dichloromethane fraction the percentage of growth reduction was 42.40% ± 0.08%. The IC50 was determined for the extracts with the highest inhibitory capacity. The IC50 obtained for C. albicans in the methanolic fraction was 475.4 μg∙mL−1 (293.5 - 770.1 μg∙mL−1) (Figure 2), and the IC50 obtained for amphotericin B (positive control) was 119.3 μg∙mL−1 (92.2 - 154.5 μg∙mL−1). Statistically significant differences were observed relative to the control group in both strains (F (2,12) = 10.06; p < 0.05 for S. cerevisiae and F (2,19) = 80.77; p < 0.05 for C. albicans).
In the current study, both extracts, from the body wall of Stichopus regalis were shown to exhibit in vitro significant antifungal activity against S. cerevisiae and C. albicans. The methanolic fraction showed the highest potential in growth inhibition tests against the two strains indicating the presence of bioactive compounds with antifungal properties. Numerous chemical and pharmacological studies carried out on several species of sea cucumbers indicate that these invertebrate contain triterpene glycoside (saponins) with bioactive properties as antifungal, anti-inflammatory, cytotoxic and others [5] [23] . Also, saponins are highly revealed in the methanolic fraction since they are a polar solvent [11] . This antifungal activity can be associated to the presence of these saponins, which have been linked to the antifungal activity in other studies concerning Holothuria edulis and Stichopus chloronotus for Candida albicans [9] , Psolus patagonicus for Cladosporium cladosporoides [24] and Holothuria polii for Aspergillus fumigatus and C. albicans [25] . Concerning C. albicans, the methanolic fraction showed the highest antifungal potential with IC50 values being lowest than those reported by Hing et al. (2007) [9] for H. edulis and S. chloronotus against C. albicans.
![]()
Figure 1. Effect of Stichopus regalis extract (1 mg∙mL−1) in the growth of S. cerevisiae (a) and of C. albicans (b). The results are represented as mean percentage of growth reduction compared to the control group ± standard deviation. # represents the statistical differences at a significant level of 5%.
3.3. Cytotoxicity Effects of Sea Cucumber Extracts in the Cell Lines MCF-7 and HepG-2
The cell viability assays showed that both in MCF-7 (Figure 3(a)) and HepG-2 (Figure 3(b)) cell lines, the dichloromethane fraction presented the highest potential, as revealed by the MTT method, in the reduction of cell viability compared to the control group. In MCF-7 the percentage was 14.13% ± 3.88% and in HepG-2 the percentage was 27.39% ± 2.21%. Statistically significant differences were observed in both cell lines for the dichloromethane fraction in MTT method (F(2,31) = 9.70; p < 0.05 for MCF-7 and F(2,27) = 50.66; p < 0.05 for HepG-2 cell lines) and no statistically significant differences were detected in Calcein-AM method in both organic fractions.
3.4. Effect of Sea Cucumber Extracts on the Inhibition of Cell Proliferation in MCF-7 and HepG-2 Cell Lines
The cell proliferation tests showed once again that in both MCF-7 (Figure 4(a)) and HepG-2 (Figure 4(b)) cell lines, the dichloromethane fraction showed the highest potential, by the MTT method, in the reduction of cell proliferation compared to the control group. In MCF-7 the proliferation reduction was 20.32% ± 4.12% and in HepG-2 was 13.79% ± 3.80%. Statistically significant differences were observed in both cell lines for the dichloromethane fraction in MTT method (F(2,31) = 11.38; p < 0.05 for MCF-7 and F(2,31) = 7.17; p < 0.05 for HepG-2 cells line).
Many chemotherapeutic agents are reported to exert their anticancer effects by inducing apoptosis of cancer cells [26] . Apoptosis, also known as programmed cell death, is characterized by typical cellular morphology and biochemical features including cell shrinkage, cytoplasm vacuolization, chromatin condensation, DNA fragmen- tation and, finally, cellular breakdown into apoptotic bodies [27] . Apoptosis is an important biological mecha- nism that contributes to the maintenance of the integrity of multi-cellular organisms that is dependent on the ex- pression of cell-intrinsic suicide machinery. The mitochondrial permeability transition is an important step in the induction of cellular apoptosis [28] . The regulation of apoptosis involves a host of molecules, in particular, the expression of Bcl-xL and Bax proteins which are altered with the induction of apoptosis [29] . In addition, sequential activation of caspases plays a central role in the execution-phase of cell apoptosis [27] . According to the results observed, the dichloromethane fraction of Stichopus regalis could inhibit both the viability and the proliferation of MCF-7 and HepG-2 cell lines by mechanisms involving the induction of apoptosis. However, further specific apoptosis studies are needed for link the apoptosis to the Stichopus regalis’ effects. The potent biological activities seen in this investigation may be attributed to the water-soluble chemicals inherently present
![]()
Figure 2. Dose-response analysis of Stichopus regalis methanolic extract against Candida albicans growth―IC50 determination.
![]()
Figure 3. Effect of Stichopus regalis extract (1 mg∙mL−1) on cell proliferation for MCF-7 (a) and HepG-2 cell lines (b) (% control), after 24 hours of incubation. Results are presented as deviation (n = 8). #―p < 0.05 represents statistically significant differences in MTT method relative to the control. C―Control group; SRM―Stichopus regalis methanolic fraction; SRD―Stichopus regalis dichloromethane fraction.
![]()
Figure 4. Effect of Stichopus regalis extract (1 mg∙mL−1) on cell proliferation for MCF-7 (a) and HepG-2 cell lines (b) (% control), after 24 hours of incubation. Results are presented as mean ± standard deviation (n = 8). #―p < 0.05 represents statistically significant differences in MTT method relative to the control. C―Control group; SRM―Stichopus regalis methanolic fraction; SRD―Stichopus regalis dichloromethane fraction.
in sea cucumbers. In general, these organisms are known to contain a high amount of good-quality protein that has been associated with beneficial effects [9] . The proteins produced from its body wall are rich in glycine, glutamic acid and arginine. These amino acids are known to stimulate production of IL-2 and B-cell antibodies. In addition, arginine can enhance cell immunity by promoting activation and proliferation of T-cells. Also, bio- active secondary metabolites, with important antitumor activity, such as triterpene glycosides, glycosaminogly- can, sulfated polysaccharides, sterols and glycosphingolipids have been reported to be present in sea cucumbers [9] [30] .
3.5. Quantification of Total Fat Content and Fatty Acid Profile
The total fat content of S. regalis was 3.63% ± 0.11%. The fatty acid profile (Table 1) revealed substantial proportions of EPA (C20:5 ω3) (12.49% ± 0.15%), DHA (C22:6 ω3) (7.35% ± 0.02%), ARA (C20:4 ω6) (19.29% ± 0.14%), Palmitic acid (C16:0) (9.43% ± 0.77%), Stearic acid (C18:0) (12.43% ± 0.83%), Vaccenic acid (C18:1 ω7) (5.63% ± 0.33%) and a ω3/ω6 ratio of 1.078.
Fatty acids are essential for life, due to their role as source of energy, membrane constituents, as well as meta- bolic and signaling mediators. Due to their positive impact on human health, alternative sources of polyunsaturated fatty acids (PUFA) are being pursued, with marine organisms being increasingly regarded as good alternatives [31] -[34] . The total fat content obtained for S. regalis was higher than for other species like Parastichopus californicus. This could be explained by seasonal variations in feeding behaviors and geographical variations [35] . Chang-Lee (1989) [35] has defined a range of total fat percentage for sea cucumbers as 0.1% - 0.9%. The fatty acid profile obtained for Stichopus regalis in the presented study showed a higher content of EPA, DHA and ARA fatty acids than what was reported by Wen et al. (2010) [36] for Stichopus herrmanni. The large concentrations of polyunsaturated fatty acids recorded as eicosapentaenoic acid (EPA; C20:5 ω3) and araquidonic acid (ARA; C20:4 ω6) are in agreement with observation by Ackman (1989) [37] , who indicated that these fatty acids are prominent in holothurians. It has been established that cold water marine species tend to accumulate higher amounts of long-chain ω3 fatty acids, particularly EPA and DHA (docosahexaenoic acid; C22:6 ω3), than species from temperate climate. Environmental temperature may affect fatty acid composition in marine organisms, with a high content of long-chain ω3 fatty acids being related to the ability to sustain membrane fluidity at lower temperatures [38] . Long-chain ω3 fatty acids are also of high interest for human nutrition as they play a crucial role in brain function, normal growth and development. These fatty acids are also associated to the reduction of coronary heart disease risk, cancer, inflammation, and arthritis [33] [34] . Similar results relating to higher concentration of EPA over DHA in deep-sea echinoderms were reported by Drazen et al. (2008) [39] , who documented ranges of 9% - 18% for EPA and 1% - 8% for DHA. Zhong et al. (2007) [10] reported very high EPA content in Cucumaria frondosa from Newfoundland, with levels ranging from 43% to 57% (w/w), and highlighted the role this fatty acid has in blood clotting because it possesses antithrombotic activity. In C. frondosa, DHA content ranged from 2% to 6% [10] . Of particular interest is the araquidonic acid (ARA) content, which was the principal ω6 polyunsaturated fatty acid detected in this study. ARA plays an important role in human health, especially in growth because it is the main precursor of eicosanoids [34] . ARA is also an important constituent of the central nervous system [40] . Interestingly, the ARA content of sea cucumbers appears to
![]()
Table 1. Fatty acid profile (%) of total lipids extracted from Stichopus regalis. Results are presented as mean ± standard deviation (n=3).
aSaturated; bMonounsaturated; cPolyunsaturated.
be consistently higher than those found in marine fish [36] [41] . The stearic (C18:0) and palmitic acid (C16:0) were the major saturated fatty acids present and its percentage were higher than those reported in Betchel et al. (2012) [41] for Parastichopus californicus.
4. Conclusion
In summary, this study provided valuable results concerning the high biotechnological and sea food potential of Stichopus regalis. A great antifungal potential was obtained, revealing possible new sources of compounds with applications for the pharmaceutical industry. The quantification of total fat and fatty acid profile demonstrated the nutritional benefits of this sea cucumber species for human health. Although these results are preliminary, further studies are required as the bioactive compounds identification and characterization. Its potential for the pharmaceutical industry or as seasonality can affect the lipid and protein content of this species.
Acknowledgements
We would like to thank Teresa Mouga as headmaster of the School of Tourism and Maritime Technology of Peniche, for providing the authorization and support to utilize the Laboratories and other facilities, necessary to conduct this work. We would also like to thank Tiago Simões and Rita Sousa for the valuable help in the lipid profile assays and Susana Mendes for all the statistical help given to this work.
Competing Interests
The authors declare that there’s no conflict of interests.
Author’s Contribution
Author contributions to the study and manuscript preparation are as follows. Conception and design: RS. Carried out the experiments: RS, SD, SP, JS and CA. Acquisition of data: RS, SD, SP, JS and CA. Analysis and interpretation: RS, SP, CA, AP, CT and RP. Drafting the article: RS, SD, SP, JS, CA, AP, CT and RP. Statistical analysis: RS. Study supervision: CT, AP and RP. All authors read and approved the final manuscript.
NOTES
*Corresponding author.