Characterization and Evaluation of Standard Enthalpy of Vaporization and Kinetic Studies of Volatile bis(N-ethyl-5-methyl-salicylaldimine)nickel(II) Complex ()
1. Introduction
Nickel films are widely used in the metallization of ferrites and as decorative and corrosion-resistant coatings. Ni and its alloy (NiSi) are also promising materials for microelectronic devices because the electrical resistivity is low and the oxidation resistance is high [1] [2] . Therefore, chemical vapour deposition (CVD) for Ni or its alloy film has been widely investigated by researchers of microelectronics and nanotechnology, because CVD is the method to deposit film with good-quality step coverage on patterned structures [3] - [5] . Ni precursor is tetracarbonylnickel(0) Ni(CO)4 [6] , whose boiling point is 43˚C and volatility is very useful as a precursor. However, it has the critical problem that it is too highly toxic. Therefore, an alternative precursor is strongly required. Until now, several Ni precursors such as a glyoximato [7] , β-diketonato [8] , or cyclopentadienyl nickel [9] [10] were utilized as an alternative. All of them are solids below 150˚C, and their volatilities are far lower than that of Ni(CO)4. In CVD, using one of them as a precursor, a high deposition temperature was needed. This paper presents the development and characterization of bis(N-ethyl-5-methyl-salicylaldimine)nickel(II) volatile precursor of nickel. The original result on this complex is presented here in order to assess the enthalpy of vaporization. This work focuses on the rapidity with which a reliable equilibrium vapour pressure could be determined with the horizontal TG/DTA thermoanalyser in a time frame which is nearly one-tenth of that required for a similar measurement with Knudsen cell mass spectrometry (KCMS) or conventional transpiration with suitable effluent gas analyser. The thermal stability and vaporization kinetics of bis(N-ethyl-5-methyl-salicylaldimine) nickel(II) under non-isothermal and isothermal TG methods are also presented.
2. Experimental Section
2.1. Materials and Development of Precursors
The chemicals liquid ammonia (Qualigens, India), NiCl2∙6H2O (purity―97%), ethylamine (purity―70%, Loba Chemie, India), ethanol (purity―99.9%, S.D. Fine, India), 5-methyl-salicylaldehyde (purity―99%, Merck, Ger- many) were used as such in the syntheses of nickel complex.
The volatility of the nickel precursor was improved by altering the ligand chemistry of the complex. The polarity of the metal-oxygen bond responsible for the ionicity of the complex could be reduced by substituting nitrogen for oxygen in the carbonyl group (C=O). Based on these considerations, the second nickel complex bis(5-methyl-salicylaldimine)nickel(II), Ni(5-me-salim)2 was prepared and was subsequently characterized. At this juncture it was decided to substitute H of =NH as shown in (III) of Scheme 1 by the ethyl group, thereby
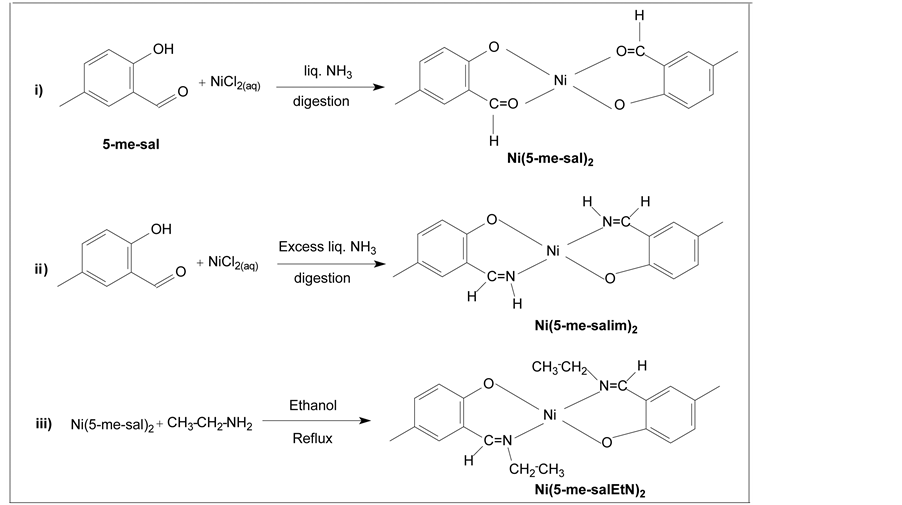
Scheme 1. Graphical representation of the Ni(5-me-saletN)2.
tuning the volatilization behaviour. This complex was considered as an ideal precursor for the CVD of metallic nickel and further characterization as well as the vapour pressure measurements was deemed to be essential, as it is imperative to know the vapour pressure of the precursor for an accurate control of the feed rate to the CVD reactor.
2.2. Characterization
The FT-IR spectrum was recorded using a Perkin-Elemer FT-IR spectrometer (RX1, FT-IR) in the region of 4000 - 400 cm−1. The C, H and O analyses were carried out by a CARBO-ERBA-11008 CHNS-O rapid elemen- tal analyzer. The electron spray ionization mass spectra of the complex was recorded on a MICRO MASS QUAT- TRO II triple quadrupole spectrometer having a JASCO PU-980 HPLC pump connected to it.
2.3. Non-Isothermal TG
The thermogram of the complex was carried out with Perkin-Elmer Pyris-Diamond TG-DTA. The thermogra- vimetric analysis was performed at a linear heating rate of 10 Kmin−1 over the temperature range of 420 - 520 K. The temperature calibration was done by the method of fixed melting points by using International Practical Temperature Scale 1968 (IPTS-68; amended in 1975) recommended standards for indium, tin, and aluminum. Approximately 3 mg of the complex was taken for the experiment. A sintered high-density alumina crucible was used as sample and reference holders, and α-alumina powder was used as the reference material. The purge gas was high purity nitrogen dried by passing through refrigerated molecular sieves (Linde 4A) at a flow rate of 12 dm3∙h−1.
2.4. Isothermal Vapour Pressure Measurements
A horizontal thermal analyser was adopted as transpiration set up for vapour pressure measurements. The block diagram of the thermal analyser, modified for its functioning in the transpiration mode is given elsewhere [11] . The details of the precise flow calibration for the carrier gas, using a capillary glass flow meter and corrections for apparent weight losses in isothermal mode, are described elsewhere [11] [12] . The sample, finely powdered using a mortar and pestle was spread on an alumina crucible and was carefully flushed with nitrogen gas at the rate of 6 dm3∙h−1 at ambient temperature. Vapour pressure measurements were carried out at 10 Kmin−1. After the temperature stabilization, subsequent changes in isothermal steps were done at a heating rate of 2 Kmin−1.
2.5. Vaporization Kinetics
The conventional non-isothermal thermogravimetric run was carried out at the heating rate of 10 Kmin−1. Among the several methods available for the kinetics evaluation of TG weight loss data Coats-Redfern was followed in the present paper to study the vaporization kinetics.
3. Results and Discussion
3.1. Characterization
The C and H analyses of the complex confirmed the assigned composition of the calculated values. The C, H and Ni analyses are provided. Anal. Calcd. for Ni(C20H24N2O2) [Ni(5-me-salEtN)2]: C, 62.66 (62.53); H, 6.26 (6.23) and Ni, 15.14 (15.18). The FT-IR absorption spectral bands of [Ni(5-me-salEtN)2] complex observed at 1612, 1543, 1328, 524, 484 cm−1 are assigned to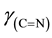 ,
, 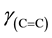 ,
, 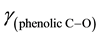 ,
, 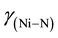 and
and 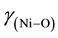 respec- tively.
respec- tively.
3.2. Thermal Properties of bis(5-methyl-salicylaldehydato)nickel(II) [Ni(5-me-sal)2]
The bis(5-methyl-salicylaldehydato)nickel(II) [Ni(5-me-sal)2]. Complex was characterized by thermogravimetry (TG) for its melting, vaporization, decomposition temperatures and volatility. However, the thermogram as shown in Figure 1 indicates the loss of two water molecules after the first step at about 417 K, and 35% residue after the second weight loss step at about 620 K; subsequent decomposition of the complex gives about 46.68% residue at the temperature of about 756 K, making it unsuitable for CVD applications. This clearly shows that
![]()
Figure 1. Non-isothermal TG curves of bis(5-methyl-salicylaldehy- dato)nickel(II); Ni(5-me-sal)2, bis(5-methyl-salicylaldimine)nickel(II); Ni(5-me-salim)2 and bis(N-ethyl-5-methyl-salicylaldimine)nickel(II); Ni(5-me-salEtN)2.
the nickel atom has been retained in the residue and not transferred into the vapour phase as evidenced by the black end residue analysis.
3.3. Thermal Properties of bis(5-methyl-salicylaldimine)nickel(II) [Ni(5-me-salim)2]
The TG trace of this complex shown in Figure 1 indicates a residue of 3.8% at 705 K after a smooth single step weight loss. The interesting inference was the enhanced thermal stability and volatility as well as the expulsion of two H2O molecules caused by the coordination of azomethine nitrogen (?CH=NH) with Ni instead of carbonyl oxygen.
3.4. Thermal Properties of bis(N-ethyl-5-methyl-salicylaldimine)nickel(II); [Ni(5-me-salEtN)2]
The non-isothermal TG run of the complex (Figure 1) revealed that the complex was thermally stable up to 490 K. The weight loss, commencing from about 490 K, occurred in a single step and ended up with total weight loss around 580 K. The fact that the complex showed a single step weight loss ending up with nil residues help- ed to identify the complex as a potential precursor for CVD of nickel films. As the objective of this investigation was to evaluate the equilibrium vapour pressure by a TG-based transpiration technique, apriori knowledge of the molecular weight of the complex was required. For this purpose, the Ni(5-me-salEtN)2 was subjected to ESI- MS analysis and its molecular mass was determined.
3.5. Determination of Standard Enthalpy of Vaporization 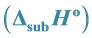 of Ni(5-me-salEtN)2
of Ni(5-me-salEtN)2
The equilibrium Vapour pressure  could be calculated using the Equation (1),
could be calculated using the Equation (1),
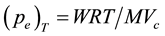 (1)
(1)
Which is derived from Dalton’s Law of partial pressures for a mixture of ideal gases, where 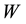 is the mass loss (mg) at an isothermal temperature caused by the flow of
is the mass loss (mg) at an isothermal temperature caused by the flow of 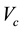 (dm3) of the carrier gas (measured at
(dm3) of the carrier gas (measured at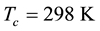 ),
), ![]() is the universal gas constant (8.314 Jmol−1∙K−1) and
is the universal gas constant (8.314 Jmol−1∙K−1) and ![]() (383.11024 g∙mol−1) is the molecular mass of the monomeric precursor vapour species. Attainment of equilibrium condition was also ascertained by the plot of isothermal time against mass loss for all the isothermal steps as shown in Figure 2 and the lines passing through origin show that equal amount vaporized at equal intervals of time. The values of observed
(383.11024 g∙mol−1) is the molecular mass of the monomeric precursor vapour species. Attainment of equilibrium condition was also ascertained by the plot of isothermal time against mass loss for all the isothermal steps as shown in Figure 2 and the lines passing through origin show that equal amount vaporized at equal intervals of time. The values of observed
![]()
Figure 2. Mass loss against time of 1 h isothermal holding.
mass loss, isothermal temperatures and the calculated equilibrium vapour pressure ![]() are listed in Table 1. The plot of
are listed in Table 1. The plot of ![]() against 1000/T (K) is shown in Figure 3. The vapour pressure
against 1000/T (K) is shown in Figure 3. The vapour pressure ![]() could be represented by the least square expression (2),
could be represented by the least square expression (2),
![]() (2)
(2)
Multiplying the slope of the expression by −2.303 R, a value of 94.2 ± 1.2 kJmol−1 was derived for the standard enthalpy of vaporization![]() . The entropy of vaporization
. The entropy of vaporization ![]() calculated from the intercept was found to be 249.4 ± 2.6 Jmol−1∙K−1.
calculated from the intercept was found to be 249.4 ± 2.6 Jmol−1∙K−1.
3.6. Determination of Activation Energy ![]() of Ni(5-me-salEtN)2
of Ni(5-me-salEtN)2
The rate constant ![]() for the vaporization enthalpy of the complex was determined in the temperature range of 480 ? 580 K for every 10% weight loss of the complex. The expression for
for the vaporization enthalpy of the complex was determined in the temperature range of 480 ? 580 K for every 10% weight loss of the complex. The expression for ![]() is given by
is given by
![]() (3)
(3)
where ![]() is the derivative of the fraction vaporized with respect to time and
is the derivative of the fraction vaporized with respect to time and ![]() is the rate constant of vaporization. By using this equation,
is the rate constant of vaporization. By using this equation, ![]() was calculated for every 10% weight loss,
was calculated for every 10% weight loss, ![]() is defined by the expression (4) as
is defined by the expression (4) as
![]() (4)
(4)
where ![]() is the percent weight at any time
is the percent weight at any time ![]() and
and ![]() and
and ![]() respectively, are the initial and final per cent sample weights [13] .
respectively, are the initial and final per cent sample weights [13] .
From the mass loss and the TG/DTA experiments, ![]() , the fraction reacted was evaluated as a function of temperature. A typical plot of
, the fraction reacted was evaluated as a function of temperature. A typical plot of ![]() versus temperature is shown in Figure 4.
versus temperature is shown in Figure 4.
The kinetics of the complex was followed by employing the Coats-Redfern approximation which gives the expression (5)
![]() (5)
(5)
The ![]() function describes the mechanism [14] of the reaction. Straight line with high-correlation coefficient and low standard deviation were selected to represent the possible mechanism. Therefore, the best fit was obtained with A2 (Avrami-Erofe’ev model) which corresponds to nucleation and growth mechanism. A plot of
function describes the mechanism [14] of the reaction. Straight line with high-correlation coefficient and low standard deviation were selected to represent the possible mechanism. Therefore, the best fit was obtained with A2 (Avrami-Erofe’ev model) which corresponds to nucleation and growth mechanism. A plot of
![]() versus 1000/T (K) gives a straight line when the correct
versus 1000/T (K) gives a straight line when the correct ![]() function is used in the equation
function is used in the equation
![]()
Table 1. Isothermal vapour pressure of Ni(5-me-salEtN)2.
![]()
Figure 3. Clausius-Clapeyron plot of Ni(5-me-salEtN)2.
![]()
Figure 4. Plot of fraction reacted ![]() versus temperature.
versus temperature.
![]()
Figure 5. Plot of ![]() versus 1000/T in K; where
versus 1000/T in K; where![]() .
.
and the plots are shown in Figure 5. From the slope, the activation energy ![]() for the vaporization of the complex yielded the value of 93.5 ± 7 k∙Jmol−1.
for the vaporization of the complex yielded the value of 93.5 ± 7 k∙Jmol−1.
4. Conclusion
The thermodynamic and vaporization kinetics of Ni(5-me-salEtN)2 complex was carried out. The TG-based transpiration technique was used to evaluate the vapour pressure data of Ni(5-me-salEtN)2. The standard enthalpy of vaporization and entropy of vaporization of the complex have been evaluated. The activation energy obtained for the Coats-Redfern method was found to agree with the value of enthalpy of vaporization. The thermal degradation mechanism for Ni(5-me-salEtN)2 system is a A2 (Avrami-Erofe’ev model) which corresponds to nucleation and growth mechanism.
Acknowledgements
The authors are grateful to the Department of Chemistry and Loyola Institute of Frontier Energy (LIFE), Loyola College, Chennai-34.
NOTES
*Corresponding author.