Transport Properties of Novel Hybrid Cation-Exchange Membranes on the Base of MF-4SC and Halloysite Nanotubes ()
1. Introduction
Perfluorinated cation-exchange Nafion membrane produced by DuPont (USA) and its analogues such as MF-4SC (LTD Plastpolymer, Russia), Dow (Dow, USA) are the most widely used in various devices (like fuel cells, electrodialyzers) and investigated ion-exchange materials [1]. Volume and surface modification of such membranes by incorporation of different additives allows to change their properties, in particular, stability and structural characteristics as well as ion and molecular transport [2]. Recently major attention of scientists has been focused on the modeling of species transport across ion-exchange membranes and a number of studies are devoted to the electromigration phenomenon. The practical application of such membranes is often based on the diffusion processes, so diffusion permeability is one of the important characteristics of ionic transport through such ion-exchange membranes [2]-[6]. In hybrid materials obtained via modification of the cation-exchange membrane matrix by inorganic and some organic compounds, dopant nanoparticles are located in the membrane pores and force out electro-neutral solution with the major part of anions [2] [7], while cations are mostly located within the Debye layer (close to the negatively charged walls of the pores) which is not affected by the dopant particle. Thus, in hybrid membranes with low dopant concentration the rate of cation transfer is higher, while the rates of anion transfer are lower than in the initial membrane [8] [9]. In recent years mathematical modeling of ion and molecular transport processes through the membranes is of great interest [10]-[14]. This article is devoted to a theoretical description of the diffusion permeability processes in a hybrid MF-4SC membrane doped by halloysite nanotubes, based on our own experimental data.
2. Theory
A boundary-value problem of diffusion of aqueous solution of a 1:1 electrolyte with concentration 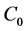 through the ion-exchange membrane into pure water was formulated and solved earlier on the basis of the Nernst-Planck transport equations [12] [15]. However those papers did not take into consideration the difference between the diffusion coefficients of cations and anions of the electrolyte in the membrane, which is crucial. This drawback has been eliminated in our recent article [16], where diffusion permeability through hybrid materials, based on MF-4SC membrane volumetrically modified by silica nanoparticles, is investigated. Meanwhile diffusive layers on both sides of the membrane (Figure 1) can significantly affect diffusion permeability. Besides that, concentration of electrolyte beyond the right diffusive layer is non-zero and slightly growing function of time. The membrane is characterized by thickness h, diffusion coefficients D+, D- (mobilities) of ions in the bulk solution and Dm+, Dm− in the membrane, respectively, absolute value of charge density of the fixed groups ρ > 0, i.e. exchange capacity, which is constant across the entire membrane, distribution coefficients γ+, γ− of cations and anions in it. The distribution coefficients were introduced in [17] to calculate the reverse osmotic separation of electrolyte solutions. They show the level of surface interaction of ions with the membrane material (γ± = exp(γ±), γ± is the interaction potential for each ion in kBT units, where kB is the Boltzmann constant, T - absolute temperature). So, as was mentioned above, we suppose that the same electrolyte with a very low concentration C0/k (k >> 1) is beyond the right side diffusive layer (x > h + δ). In the limiting case, k → ∞, we have got pure water in 4-th region (Figure 1).
through the ion-exchange membrane into pure water was formulated and solved earlier on the basis of the Nernst-Planck transport equations [12] [15]. However those papers did not take into consideration the difference between the diffusion coefficients of cations and anions of the electrolyte in the membrane, which is crucial. This drawback has been eliminated in our recent article [16], where diffusion permeability through hybrid materials, based on MF-4SC membrane volumetrically modified by silica nanoparticles, is investigated. Meanwhile diffusive layers on both sides of the membrane (Figure 1) can significantly affect diffusion permeability. Besides that, concentration of electrolyte beyond the right diffusive layer is non-zero and slightly growing function of time. The membrane is characterized by thickness h, diffusion coefficients D+, D- (mobilities) of ions in the bulk solution and Dm+, Dm− in the membrane, respectively, absolute value of charge density of the fixed groups ρ > 0, i.e. exchange capacity, which is constant across the entire membrane, distribution coefficients γ+, γ− of cations and anions in it. The distribution coefficients were introduced in [17] to calculate the reverse osmotic separation of electrolyte solutions. They show the level of surface interaction of ions with the membrane material (γ± = exp(γ±), γ± is the interaction potential for each ion in kBT units, where kB is the Boltzmann constant, T - absolute temperature). So, as was mentioned above, we suppose that the same electrolyte with a very low concentration C0/k (k >> 1) is beyond the right side diffusive layer (x > h + δ). In the limiting case, k → ∞, we have got pure water in 4-th region (Figure 1).
Inside each of diffusive layers membrane, −δ < x < 0 and h < x < h + δ, the fluxes of ions can be represented in the following form:
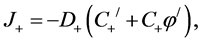 (1)
(1)
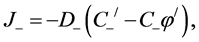 (2)
(2)
![]()
Figure 1. A scheme of the diffusion transfer of ions through a charged single layer membrane: 0 and 4―regions of intensive steering with constant concen- tration of solution; 1 and 3―left and right diffusive layers; 2―membrane.
where the bar at the right top of the symbol stands for a derivative in x, and  -is a non-dimensional electric potential in the
-is a non-dimensional electric potential in the 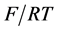 units (F is the Faraday constant, R is the universal gas constant, T-absolute temperature).
units (F is the Faraday constant, R is the universal gas constant, T-absolute temperature).
Inside the membrane, 0 < x < h, the fluxes are:
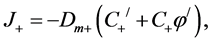 (3)
(3)
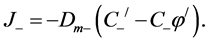 (4)
(4)
The boundary conditions of equality of the electrochemical potentials are written at the interfaces x = 0 and x = h of cation-exchange membrane [12] [13] [18]:
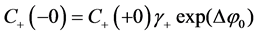 (5)
(5)
 (6)
(6)
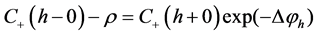 (7)
(7)
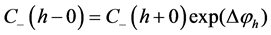 (8)
(8)
where C± are concentrations of ions, 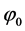 and
and 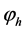 are drops of the electric potential at the membrane-electrolyte interfaces, x=0 and x=h, accordingly. To close the system of Equations (1)-(8) it should be complemented by the conditions of electroneutrality:
are drops of the electric potential at the membrane-electrolyte interfaces, x=0 and x=h, accordingly. To close the system of Equations (1)-(8) it should be complemented by the conditions of electroneutrality:
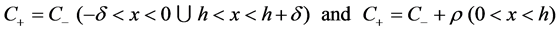 (9)
(9)
as well as the absence of electric current, which for the 1:1 electrolyte reads:
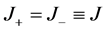 (10)
(10)
where J is the flux density of ions, and continuities of ion concentrations and electric potential on the external boundaries of diffusive layers:
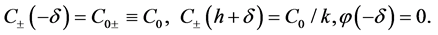 (11)
(11)
For purpose of convenience let us introduce dimensionless parameters:
![]()
![]()
![]() (12)
(12)
It should be noticed that two last notations (![]() and
and![]() ) are the averaged coefficients of the cation/anion pair diffusion in a bulk solution and inside the membrane correspondingly. The solution of the boundary value problem (1)-(11), which can be obtained by standard methods, yields the implicit algebraic expression for the diffusion permeability
) are the averaged coefficients of the cation/anion pair diffusion in a bulk solution and inside the membrane correspondingly. The solution of the boundary value problem (1)-(11), which can be obtained by standard methods, yields the implicit algebraic expression for the diffusion permeability ![]() of an arbitrary 1:1 electrolyte through the membrane:
of an arbitrary 1:1 electrolyte through the membrane:
![]() (13)
(13)
where![]() . If
. If ![]() (neglecting of diffusive layers) and k → ∞ (pure water in 4-th region, Figure 1) then we immediately get the explicit formula for diffusive permeability which was derived earlier [16]:
(neglecting of diffusive layers) and k → ∞ (pure water in 4-th region, Figure 1) then we immediately get the explicit formula for diffusive permeability which was derived earlier [16]:
![]() (14)
(14)
where notation ![]() is averaged coefficient of equilibrium distribution of the pair of ions of the 1:1
is averaged coefficient of equilibrium distribution of the pair of ions of the 1:1
electrolyte, which was first introduced in [17]. In the limit, ![]() , that means infinitely large concentration of electrolyte (
, that means infinitely large concentration of electrolyte (![]() ), from Formula (13) we get maximal (asymptotic) possible value of the diffusion permeability coefficient:
), from Formula (13) we get maximal (asymptotic) possible value of the diffusion permeability coefficient:
![]() (15)
(15)
In other limit, ![]() , we have
, we have ![]() and
and![]() , as it is follows again from (13) under small electrolyte concentration:
, as it is follows again from (13) under small electrolyte concentration:
![]() (16)
(16)
The behavior of the diffusion permeability of a 1:1 electrolyte, passing through uniformly charged cation- exchange membranes into water, is presented in Figure 2. Usually, for simplicity, we can neglect value ![]() in Equations (13), (15) and (16) because of large
in Equations (13), (15) and (16) because of large![]() .
.
Note also, that ![]() is “apparent” value of the coefficient of integral diffusion permeability of the membrane while “intrinsic” value of that coefficient is
is “apparent” value of the coefficient of integral diffusion permeability of the membrane while “intrinsic” value of that coefficient is
![]()
so, consequently,
![]()
and it is associated with three-layer membrane system. When ![]() and
and ![]() (it is usual situation) these coefficients are approximately equal:
(it is usual situation) these coefficients are approximately equal:![]() .
.
When ![]() from (14) we get the following approximate formula
from (14) we get the following approximate formula
![]() (17)
(17)
![]()
Figure 2. Dependence of the diffusion permeability of a cation-exchange membrane on electrolyte concentration.
which is convenient in order to find preliminary values of effective averaged diffusivity ![]() in the membrane and effective exchange capacity
in the membrane and effective exchange capacity![]() .
.
It should be noted that parameters![]() ,
, ![]() and
and ![]() unlike
unlike![]() ,
, ![]() and
and ![]() could not be determined through direct independent experiments. Parameters
could not be determined through direct independent experiments. Parameters![]() ,
, ![]() ,
, ![]() and, consequently, ion diffusivities
and, consequently, ion diffusivities![]() ,
,
![]() for any 1:1 electrolyte could be calculated via the method of least squares using Equations (13), (14) or (17) from the experimental values of the exchange capacity of swollen membrane
for any 1:1 electrolyte could be calculated via the method of least squares using Equations (13), (14) or (17) from the experimental values of the exchange capacity of swollen membrane![]() , averaged ion diffusivity
, averaged ion diffusivity ![]() in a bulk electrolyte solution (for example,
in a bulk electrolyte solution (for example, ![]() for NaCl), ratio
for NaCl), ratio ![]() and experimental dependence of
and experimental dependence of ![]() for the 4 - 6 values of concentration of a 1:1 electrolyte ( 0.1 M ≤ C0 ≤ 1 M ). The thickness of the membrane, h, can be measured directly by micrometer and the thickness of diffusive layer can be evaluated from known formula [19]:
for the 4 - 6 values of concentration of a 1:1 electrolyte ( 0.1 M ≤ C0 ≤ 1 M ). The thickness of the membrane, h, can be measured directly by micrometer and the thickness of diffusive layer can be evaluated from known formula [19]:
![]() (18)
(18)
where ![]() is kinematical viscosity of electrolyte solution and
is kinematical viscosity of electrolyte solution and ![]() is rotational velocity of a stirrer.
is rotational velocity of a stirrer.
3. Experiment
Original perfluorinated cation-exchange and two hybrid membranes with pure aluminosilicate (halloysite) nanotubes were prepared by casting method. For the proposed method we used a solution of sulfopolymer of the MF-4SC membrane in lithium form (7.1%wt. in dimethylformamide solution, exchange capacity was 0.98 mg- eq/g).
To modify the polymer solution it was mixed with the sample of halloysite clay nanotubes in an amount of 5 and 8%wt. to the weight of the pure polymer and homogenized by mechanical stirring. Halloysite clay is natural tubule material formed by rolled kaolin sheets. Halloysite is alumosilicate and is chemically identical to kaolin although usually contains minor amount (less than 1 wt%) of iron ions. Typically 10 - 15 alumosilicate layers roll into the cylinder [20] [21]. Surface of halloysite tubes is silica and its innermost is alumina, providing strong negative zeta-potential of approximately −30 mV on the tube surface and +25 mV in the tube innermost in aqueous dispersions at normal pH. Halloysite tubes’ diameter is of 40 - 60 nm with inner lumen diameter of 10 - 15 nm and length of 1500 ± 500 nm.
The suspension of the polymer with halloysite nanotubes was placed in a Petri dish and spreaded uniformly with a rotating table, and dried in air at room temperature for 24 hours, and then in sequence at 100˚C for 1 hour, at 120˚C for 1 hour, at 130˚C for 1 hour, at 140˚C for 1 hour and finally at 120˚C for 4 hours to remove the solvent gradually and form a film of membrane. Thereafter, the film was removed carefully from the surface.
The resultant hybrid membranes were visually homogeneous throughout the sample area but the slight turbidity was observed. The thicknesses of the resultant membranes were 390 (original membrane), 310 (5%wt. of halloysite) and 290 (8%wt. of halloysite) microns. In the analysis of SEM and AFM micrographs it is worthy to notice that the nanotubes are uniformly distributed on the surface of the membrane. The electron and atomic- force microscopy results of synthesized hybrid membranes are shown in Figure 3(a) and Figure 3(b). We can conclude that aluminosilicate nanotubes and micro-cavities are observed on both images. The cavities size inreases as halloysite concentration increases, thus agreeing with Berns [22].
After the synthesis, the membranes were washed with water and then equilibrated with HCl, LiCl, or NaCl solutions at specified concentrations. Following this, its transport characteristics were investigated with experimental methods described in [23]. The specific electrical conductivity of the membranes (![]() , S/m), in the case of NaCl and HCl solutions at 0.1 M concentration, was calculated from resistance data, which were measured as the active part of the impedance at an alternating-current frequency of 50 - 500 kHz by the mercury-contact method. The diffusion transmembrane flux, J, and the integral coefficient of diffusion permeability, Р, were determined from the diffusion of the electrolyte solution (NaCl) into pure water in a two-chambered cell equipped with platinized platinum electrodes and two magnetic stirrers (circular velocity of stirring was equal to 200 1/s). All experiments were carried out under isothermal conditions at 25˚C. These experiments were conducted in laboratory of Prof. Kononenko N.A. at Kuban State University (Russia). The error in determining the transport characteristics on a single membrane sample did not exceed 3% - 5%. Experimental values of diffusion permeability are shown by bold points in Figures 4(a)-(c).
, S/m), in the case of NaCl and HCl solutions at 0.1 M concentration, was calculated from resistance data, which were measured as the active part of the impedance at an alternating-current frequency of 50 - 500 kHz by the mercury-contact method. The diffusion transmembrane flux, J, and the integral coefficient of diffusion permeability, Р, were determined from the diffusion of the electrolyte solution (NaCl) into pure water in a two-chambered cell equipped with platinized platinum electrodes and two magnetic stirrers (circular velocity of stirring was equal to 200 1/s). All experiments were carried out under isothermal conditions at 25˚C. These experiments were conducted in laboratory of Prof. Kononenko N.A. at Kuban State University (Russia). The error in determining the transport characteristics on a single membrane sample did not exceed 3% - 5%. Experimental values of diffusion permeability are shown by bold points in Figures 4(a)-(c).
![]()
![]() (a) (b)
(a) (b)
Figure 3. SEM (a) and AFM (b) micrographs of the surface of hybrid MF-4SC membrane obtained by casting method (aluminosilicate nanotubes and micro-cavities are observed on both images).
![]() (a)
(a)![]()
![]() (b) (c)
(b) (c)
Figure 4. Experimental (bold points) and theoretical (curves) dependencies of the diffusion permeability of original (a) and hybrid MF-4SC/halloysite membranes with 5% wt; (b) and 8% wt; (c) content of nanotubes.
4. Results and Discussion
Using simplified formula (17) and Mathematica 9 software with the help of least square method from experimental data we found two main physico-chemical parameters of the membranes: effective averaged mobility of Na+ and Cl− ions (![]() ) and their effective exchange-capacity (
) and their effective exchange-capacity (![]() ) (Table 1).
) (Table 1).
It is clear that 5% wt. hybrid membrane is much better than 8% wt. membrane because it has larger ion-ex- change capacity and lower diffusion permeability even in comparison with original MF-4SC membrane. Probably,
![]()
Table 1. Calculated values of physicochemical parameters.
adding 8% wt. of halloysite nanotubes leads to strong irreversible degradation of perfluorinated cation-ex- change membrane. This hypothesis is proved by increase in electrical conductivity (Table 1) and enlargement of microcavities inside the membrane (Figure 3). Since the exchange capacity ![]() of the original (unmodified) membrane has been known (0.98 M), its averaged coefficient
of the original (unmodified) membrane has been known (0.98 M), its averaged coefficient ![]() of equilibrium distribution is equal to 0.67 and averaged mobility
of equilibrium distribution is equal to 0.67 and averaged mobility ![]() of ionic pair is 10.7
of ionic pair is 10.7![]() , that is in a good correlation with [16] [18].
, that is in a good correlation with [16] [18].
Figure 4 shows good correspondence between experimental and theoretical behavior of diffusion permeability P as function of NaCl electrolyte concentration for all three investigated membranes even in the case of application of simplified Formula (17). Utilizing implicit Equation (13) will allow us to clarify values of physicochemical parameters of the system and find individual diffusivities of ions inside a membrane.
So, in this article we continued to develop a method of quantitative evaluation of physicochemical parameters of the system electrolyte solution―ion-exchange membrane―water (averaged and individual diffusion coefficients and averaged distribution coefficient of ion pairs in the membrane). The parameters of hybrid cation-ex- change MF-4SC membranes with different halloysite concentration were obtained by this method with the use of experimental data on the diffusion permeability of NaCl electrolyte. The results allow us to evaluate the effect of membrane modification and to choose optimal compositions for hybrid membranes.
Acknowledgements
This work is partially supported by the Russian Science Foundation (grant RSF No. 14?19?01045) and CRDF- Global (project # FSAX-14-60158-0).